Figure 1.
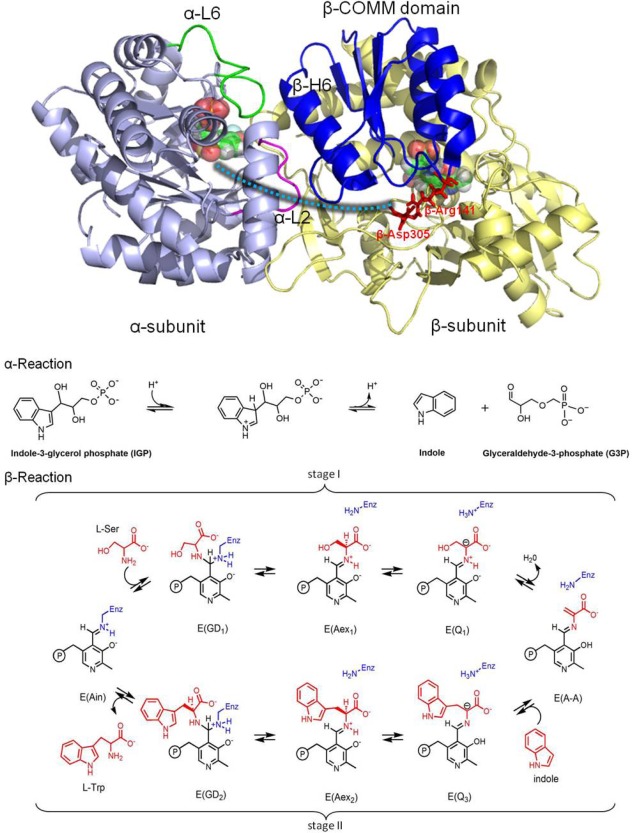
Overall structure and chemical reactions of tryptophan synthase (TRPS). (top) TRPS is composed of an α‐subunit (purple) and β‐subunit (yellow). The two ligands binding to each subunit are shown in bead representation. The open, partially closed, and fully closed conformations of the α‐subunit are controlled by αL2 (pink), αL6 (green), and βHelix‐6 of the COMM domain (blue). The residues βArg141 and βAsp305, highlighted in red, are associated with the open and closed β‐site conformations. The tunnel used to channel indole from the α‐site to the β‐site is marked as a cyan dashed line. (bottom) The α‐ and β‐reactions of TRPS.
