Figure 1.
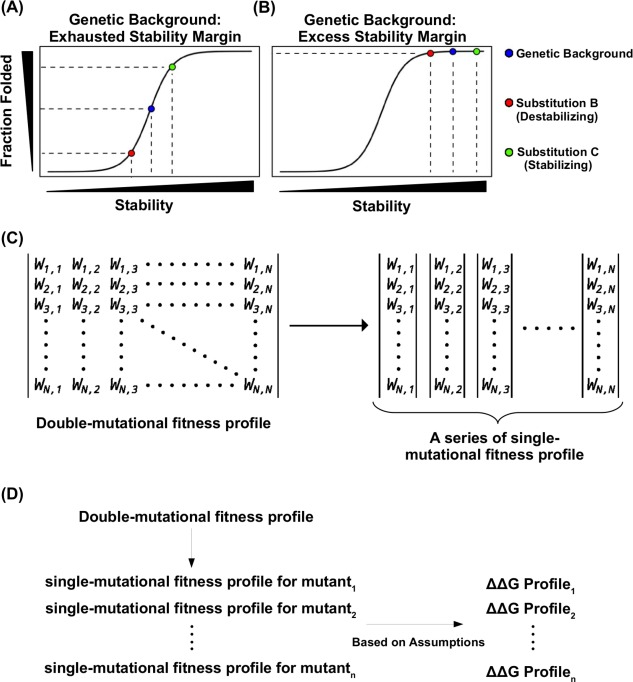
Conceptual basis for studying protein stability from functional measurement. (A, B) A schematic representation of the nonlinear relationship between mutant stability and protein folding under genetic backgrounds with different stabilities is shown for (A) a destabilizing genetic background, in which the protein is partially unfolded, and (B) a stable genetic background, in which the protein is fully folded (native state). Blue represents the genetic background, red represents a destabilizing substitution on the genetic background, green represents a stabilizing substitution on the genetic background. (C) A double‐substitution functional profile can be partitioned into individual single‐substitution functional profile for different genetic backgrounds. The double‐substitution functional profile is shown as a symmetric matrix. The fitness value of each mutant was indicated by , where i and j indicates the substitution. When i equals j, it represents a single substitution. (D) A diagram shows the logical flow of computing G from a double‐substitution functional profile. G for individual single substitution can be computed from the functional profile of a given genetic background. Nonetheless, several assumptions are involved in the computing of G from functional profile. As a result, only those genetic backgrounds that satisfy the assumptions would allow accurate calculation of G from the functional profile.
