Figure 5.
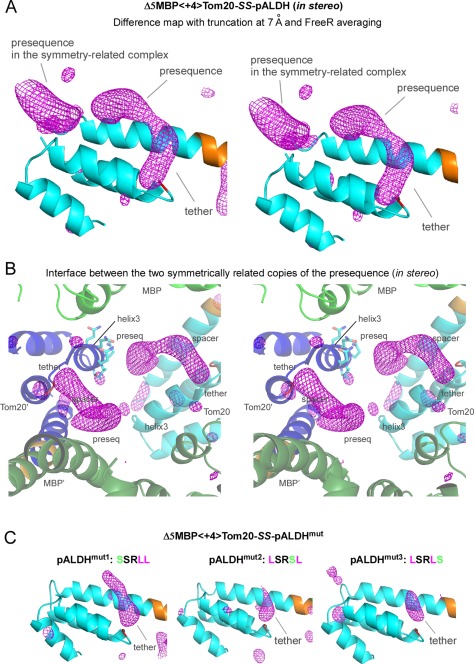
Electron densities in the CCFS corresponding to the presequence peptide and disulfide tether in the binding site of Tom20. (A) Stereo view of the truncated and FreeR‐averaged difference map of Δ5MBP<+4>Tom20‐SS‐pALDH, contoured at +3σ. The electron densities in the binding site of Tom20 were improved by the averaging of 20 maps calculated with different free test sets, as compared with the map without FreeR averaging in Figure 3(C). Tom20 and the 4‐residue spacer are colored cyan and orange, respectively. (B) Stereo close‐up view of the interface between two symmetrically related copies of the elongated electron density. MBP and Tom20 are colored green and cyan in one molecule, and dark green and blue in another molecule, respectively. The side chains of the helix3 of one Tom20 structure are shown in a stick model. (C) Effects of the essential leucine substitutions in pALDH on the electron density in the CCFS. These difference maps were generated and drawn in exactly the same way as in (A). Only the tether parts remained visible in the CCFS.
