Figure 5.
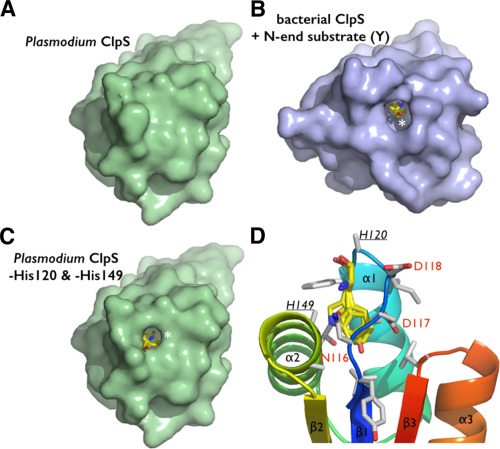
Steric adequacy and adaptability of the N‐degron specific pocket of Plasmodium ClpS. (A) Solvent accessible surface representation of the Pfal‐ClpS showing the absence of accessible binding cavity in contrast with the bacterial ClpS. (B) shown bound to a tyrosine N‐end degron (yellow sticks and white asterisk). (C) Side chains of residues H120 and H149 conceal a N‐degron binding pocket in Pfal‐ClpS. Solvent accessible surface representation of Pfal‐ClpS where side chains of residues H120 and H149 (underlined) have been omitted (all side chain atoms except the Cα where removed) is shown and reveals a pocket large enough to accommodate N‐end rule residues such as tyrosine (yellow sticks and white asterisk). (D) The Pfal‐ClpS binding pocket is shown with 4 distinct side chains (Y, F, W, and L) bound as observed in bacterial ClpS/peptide complexes (PDBs 3DNJ, 3G19, 3GQ1, and 3GW1).11, 12 Labeled residues delineate the hydrophobic pocket shown in the same orientation as in [Fig. 3(B)].
