Figure 3.
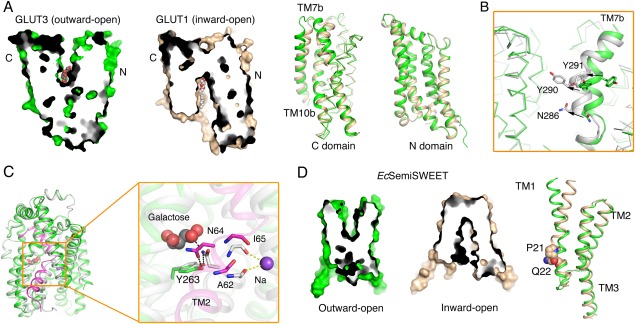
The alternating access mechanism of GLUTs, vSGLT, and SemiSWEET. (A) Structural comparison of the outward‐open GLUT3 and the inward‐open GLUT1 (PDB accession codes 4ZWC and 4PYP, respectively). Shown on the left are the cut‐open views of the surface representation of GLUT1 and GLUT3. Structural superposition of their C domains reveals local shifts in TM7b and TM10b, while the N domains remain nearly unchanged. (B) Local shifts of TM7b result in the conformational changes of GLUT3 from outward‐open to ‐occluded. The superposition of outward‐open GLUT3 (green) with the outward‐occluded (white) one reveals pronounced conformational changes of TM7b (PDB accession code for outward‐occluded GLUT3: 4ZWB). (C) Local rearrangement of TM2 results in the conformational switch from inward‐occluded to inward‐open of vSGLTs. The vSGLTs in the inward‐facing and inward‐open state are colored white and green, respectively. The TM2 of vSGLT in the inward‐open structure is highlighted by magenta. The rearrangement of the key residues (Ala62/Asn64/Ile65) in TM2 and the intracellular gate Try263 is shown on the right. (D) Structural comparison of EcSemiSWEET in the outward‐open and inward‐open states (PDB accession code 4X5N). The superposition of monomers of EcsemiSWEET in different conformations suggests that conserved PQ motif in each protomer serves as a hinge for the conformational bending of TM1.
