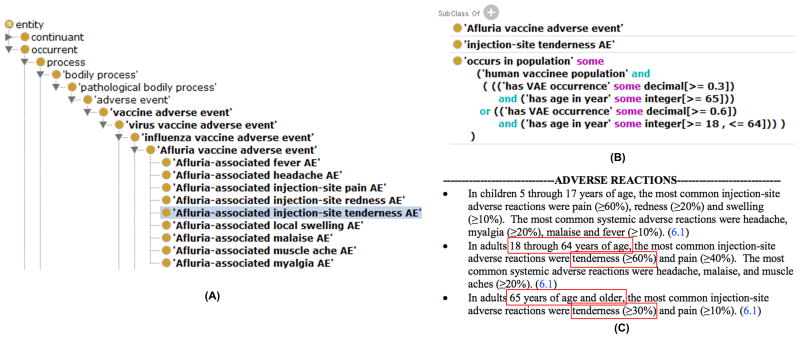
Figure 4. OVAE representing Afluria VAEs reported in FDA vaccine package insert.
(A) The hierarchical structure of Afluria VAEs represented in OVAE. (B) OVAE ontological representation of ‘Afluria-associated injection-site tenderness AE’ based on two age groups. (C) Afluria adverse reactions recorded in the FDA package insert document. The (A) and (B) subfigures were screenshots of OVAE using the Protégé OWL editor. The text from (C) was retrieved from the FDA package insert document of the Afluria vaccine. The red color box highlighted terms in (C) represents different age groups and percentage rate (i.e., occurrence) of vaccinees who are likely have to the tenderness adverse event (AE). The occurrence rates of tenderness AE under two different age groups are ontologically represented in OVAE (B). OVAE also represents other Afluria-associated AEs using the same strategy (A). AE: Adverse Event; OWL: Web Ontology Language; OVAE: Ontology of Vaccine AEs.