Abstract
The impact of logging and subsequent recovery after logging is predicted to vary depending on specific life history traits of the logged species. The Eco-gene simulation model was used to evaluate the long-term impacts of selective logging over 300 years on two contrasting Brazilian Amazon tree species, Dipteryx odorata and Jacaranda copaia. D. odorata (Leguminosae), a slow growing climax tree, occurs at very low densities, whereas J. copaia (Bignoniaceae) is a fast growing pioneer tree that occurs at high densities. Microsatellite multilocus genotypes of the pre-logging populations were used as data inputs for the Eco-gene model and post-logging genetic data was used to verify the output from the simulations. Overall, under current Brazilian forest management regulations, there were neither short nor long-term impacts on J. copaia. By contrast, D. odorata cannot be sustainably logged under current regulations, a sustainable scenario was achieved by increasing the minimum cutting diameter at breast height from 50 to 100 cm over 30-year logging cycles. Genetic parameters were only slightly affected by selective logging, with reductions in the numbers of alleles and single genotypes. In the short term, the loss of alleles seen in J. copaia simulations was the same as in real data, whereas fewer alleles were lost in D. odorata simulations than in the field. The different impacts and periods of recovery for each species support the idea that ecological and genetic information are essential at species, ecological guild or reproductive group levels to help derive sustainable management scenarios for tropical forests.
Keywords: Dipteryx odorata, Jacaranda copaia, gene flow, genetic diversity, Amazon forest, selective logging
Introduction
In Brazil, it is recognised that use of reduced impact logging techniques are essential for sustainable forest management (Sist et al., 2003; Sist and Ferreira, 2007). Although reduced impact logging mitigates deleterious impacts of timber harvesting, it does not address many issues related to sustaining timber yields or sustainable forest management (Putz et al., 2008). Studies where timber stocks have been monitored or modelled to show that reduced impact logging alone does not guarantee that timber levels will be maintained in successive cutting cycles, and that biodiversity and ecosystem functions can be compromised (De Graaf, 2000; Kammesheidt et al., 2001; Fredericksen et al., 2003; Sist et al., 2003; van Gardingen et al., 2003; Dauber et al., 2005; van Gardingen et al., 2006; Sist and Ferreira, 2007; Pena-Claros et al., 2008; Sebbenn et al., 2008).
Currently, under Brazilian legislation (IN N°5, 2006), selective logging of an area may involve removal of up to 90% of the trees of any timber species (unless there are technical specifications to support otherwise) with a diameter at breast height (d.b.h.) greater than 50 cm and logging cycles of 30 years. Assessment of sustainable forest management options requires long-term testing with a range of logging intensities, varied cutting cycles and minimum cutting diameters to understand the effects on the population of remaining trees (Sist et al., 2008). The majority of studies on the long-term impacts of logging in tropical trees have been based on field experiments (Silva et al., 1989; Alder and Silva, 2000), although increasing numbers are based on modelling (for example, Barreto et al., 1998; Pinard and Cropper, 2000; Schulze et al., 2005; Degen et al., 2006; Valle et al., 2006; van Gardingen et al., 2006; Sebbenn et al., 2008). For field-based experiments, data cover relatively short time intervals, before and after logging, that is incapable of detecting the long-term impacts of logging, particularly over successive logging cycles. Modelling approaches offer the possibility to study the complexity of tropical trees over successive logging cycles. Simulations can quantify the impacts of logging (for example, on growth rate, reproductive success and genetic structure of remaining trees), and explore different scenarios to ensure the sustainable management of a species. Eco-gene is the first model for tropical forests that integrates both ecological and genetic data in the simulation of forest management practices (Degen et al., 2002). In this study, we compared the results from modelling with empirical post-logging genetic data from microsatellite markers.
This study forms part of the Dendrogene project (genetic conservation within managed forests in the Amazon). An important project component is the integration of genetic data with growth, regeneration and other ecological data of timber species in a simulation model analysis, with different scenarios and intensities of logging, to produce information that can influence policy and management practices to achieve sustainable forest management.
The objectives of this study were: (1) to integrate existing ecological and genetic data into the Eco-gene programme to evaluate the long-term sustainability (from genetic, sustained growth and yield perspectives) of different selective logging scenarios for two species, D. odorata and J. copaia, with contrasting ecological characteristics; (2) to perform a short-term validation of the Eco-gene model through a comparison of real data from one logging event (before and after logging; Vinson, 2009) with the outputs of simulated data.
Materials and methods
Study species
Dipteryx odorata (Fabaceae) is a slow growing climax tree that occurs at very low densities. It has asynchronous flowering within populations with two flowering events per year: in the dry season (ca 35% trees flowering with asynchrony) and wet season (ca 8% trees flowering with asynchrony; Maués, 2006). The hermaphrodite flowers attract over 40 pollinator species, visitors and nectar robbers. Pollinators include 15 bee species (Apidae), 2 beetle species (Cnemida), 2 hummingbird species and, occasionally, butterflies from three families (Papilionidae, Hesperiidae, Nymphalidae; Maués, 2006), with fruit dispersal, primarily by bats. Controlled pollinations suggest that D. odorata is an obligate outcrosser, with a late-acting self-incompatibility system (Maués, 2006).
Jacaranda copaia (Bignoniaceae) is a pioneer tree widespread across the Amazon, where it colonizes gaps in lowland moist and wet forest. J. copaia trees have an annual flowering pattern, with up to 97% of monitored trees flowering each year (Maués et al., 2008). In the National Forest of Tapajós, the flowers of J. copaia attract around 40 species of bees, wasps, butterflies and hummingbirds, but the main pollinators are medium-sized solitary bees, Euglossa spp. and Centris spp. (Maués et al., 2008). The mature fruit contains up to 250 winged seeds, which are wind-dispersed. Controlled pollinations suggest that J. copaia is a self-incompatible and obligate outcrosser (Maués et al., 2008).
Eco-gene model
The Eco-gene simulation model was originally developed to investigate long-term temporal and spatial dynamics of the genetic structure of temperate tree populations (Degen et al., 1996) and then modified for the greater complexities of tropical forest species (Degen et al., 2002). The model combines elements of population genetics (allele and genotype frequencies), demographic dynamics, growth and management. Overlapping or separate generations can ‘be created' and different processes such as pollen flow, mating system, phenology and selection incorporated. The model simulates one species at a time, without accounting for competition between species. The model defines an area of forest with sufficient trees (ca 250 trees) to look at long-term genetic processes. For a detailed description of the model see Degen et al. (2006).
Input data
The input data came from the Intensive Sample Plot (ISP) study site in the Tapajós National Forest (details in Vinson, 2009), and consist of a description of the population (number of trees, plot area), spatial position (x–y coordinates), d.b.h. and multilocus genotype of each tree. Multilocus genotypes were input for seven microsatellite loci for D. odorata (Vinson et al., 2009) and five microsatellite loci for J. copaia (Vinson et al., 2008). For J. copaia, the original ISP population (256 trees ⩾10 cm d.b.h. in 200 ha) was used. For D. odorata, the ISP contained only 77 trees (⩾10 cm d.b.h. in 500 ha) requiring use of a Data Generation Engine (Dendrobase) subprogramme to generate a larger model population to match the actual demographic and genetic parameters from the field study. The inputs for the subprogramme are allele frequencies, genetic diversity, fixation index, number of trees per ha in different d.b.h. classes, size of the area or number of trees to simulate. A model population of 256 adult D. odorata trees (that is, ⩾10 cm d.b.h.) was generated to match the population size of J. copaia so both species could be compared.
Eco-gene was initially run for 20 years to simulate generation of a cohort of juveniles below 10 cm d.b.h., which were then added to the original input for both species. Although D. odorata is a tetraploid, and the Eco-gene model runs the genetic analysis as a diploid, the results can be used to infer how diploid species with similar ecological and reproductive characteristics as D. odorata may respond under different management practices.
Parameter configuration
The model requires a set of ecological, reproductive and genetic data as input. The parameters used in the model for this study were based on data from the ISP population in the Tapajós National Forest. The original field inventories provided density and size class distributions for trees, as well as an aggregation index specifying the spatial clumping of trees, for input as demography parameters (Table 1). The mating system and gene flow results for D. odorata and J. copaia (Vinson, 2009), together with studies of reproductive biology (Maués, 2006), were used as outcrossing, pollination and seed dispersal input parameters (Table 1).
Table 1. Input values for J. copaia and D. odorata Eco-gene simulations of different logging scenariosAbbreviation: d.b.h., diameter at breast height.
| Species | Parameter | J. copaia | D. odorata |
|---|---|---|---|
| Time scale | Number of years simulated | 420 | 305 |
| Number of repetitions | 100 | 100 | |
| Growth | Mean annual diameter growth rate (cm) | 0.54 | 0.35 |
| Annual growth rate standard deviation (cm) | 0.25 | 0.2 | |
| Temporal autocorrelation growth | 0.53 | 0.46 | |
| Maximum diameter (cm) | 106 | 190 | |
| Maximum height (m) | 40 | 40 | |
| Demography (N ha−1) | Density diameter class 0–10 | 1 | 0.05 |
| Density diameter class 10–20 | 0.28 | 0.02 | |
| Density diameter class 20–30 | 0.33 | 0.018 | |
| Density diameter class 30–40 | 0.2 | 0.036 | |
| Density diameter class 40–50 | 0.15 | 0.018 | |
| Density diameter class 50–60 | 0.15 | 0.018 | |
| Density diameter class 60–70 | 0.11 | 0.0216 | |
| Density diameter class 70–80 | 0.04 | 0.036 | |
| Density diameter class 80–90 | 0.04 | 0.0144 | |
| Density diameter class 90–100 | 0.01 | 0.0288 | |
| Density diameter class 100–110 | 0.01 | 0.0216 | |
| Density diameter class 110–120 | 0.018 | ||
| Density diameter class 120–130 | 0.0144 | ||
| Density diameter class 130–140 | 0.0036 | ||
| Density diameter class 140–150 | 0.0036 | ||
| Density diameter class 150–160 | 0.0072 | ||
| Density diameter class 160–170 | 0.0072 | ||
| Density diameter class 170–180 | 0.0036 | ||
| Density diameter class 180–190 | 0.001 | ||
| Phenology | Percentage of flowering adults—minimum | 80 | 62 |
| Percentage of flowering adults—maximum | 93 | 76 | |
| Minimum d.b.h. of flowering trees mean (cm) | 23 | 39 | |
| Minimum d.b.h. of flowering trees standard deviation (cm) | 5 | 2 | |
| Flowering pattern | Annual | Annual | |
| Start of flowering phase (mean, Julian day) | 305 | 289 | |
| Start of flowering phase (standard deviation) | 10 | 30 | |
| Length of flowering phase (mean in days) | 16 | 16 | |
| Length of flowering phase (standard deviation) | 8 | 5 | |
| Pollination | Self-incompatibility—minimum | 0.9 | 0.9 |
| Self-incompatibility—maximum | 0.8 | 0.8 | |
| Flight distance pollinator—maximum (m) | 1500 | 1500 | |
| Flight distance pollinator standard deviation (m) | 1000 | 1000 | |
| Seed dispersal | Proportion wind dispersal | 0.5 | 0 |
| Proportion gravity dispersal | 0 | 0 | |
| Proportion dispersal by small birds or bats | 0.5 | 1.0 | |
| Small birds or bats minimum distance (m) | 100 | 100 | |
| Small birds or bats maximum distance (m) | 1000 | 2000 |
Forest management parameters include logging cycle in years, the first and last year of treatment, the minimum cutting diameter at breast height (MCD) and the proportion of remaining trees that have at least the MCD. The model implements post-logging mortality a year after each logging episode. For both species, the following values for post-logging mortalities were used: 0–10 cm d.b.h. 20% 11–20 cm 15% 20–50 cm 10% (Gourlet-Fleury et al., 2004).
Control and selective logging scenarios
The control scenario, in which no logging is carried out, is the basis for evaluating the impact of all logging scenarios. As the aim of this study was to test the effects of logging on demographic and genetic parameters, it was important that populations show demographic stability during control simulations before testing the effects of logging. Although research has shown that tropical lowland forests may not necessarily be in equilibrium, a review of 14 large-scale tropical forest plots showed that in most forests, size distributions are much closer to predictions of demographic equilibrium, with intersite variation in size distributions explained partly by intersite variation in growth and mortality (Muller-Landau et al., 2006). Assuming that the studied population is at equilibrium, the control simulation should not show significant demographic change over the simulation period. However, during the control simulations, even without logging, there was a decline in the number of trees and basal area for both species. Therefore, to ensure an equilibrium population for each species, testing and adjustments of the control scenario were made based on the demographic parameters. To achieve demographic stability for the J. copaia population, it was necessary to run the simulation for 119 years. The population obtained after 119 years was then used as a starting point for logging simulations. For D. odorata control simulations showed that the number of trees and basal area of the control population declined over time because of the model underestimating the population density. To overcome this problem, density parameters were tested by multiplying original values by factors of 1.1, 1.2, 1.3, 1.4 and 1.5; 1.4 giving the best results in terms of a stable and realistic population density. With this modification, the control scenario of D. odorata stabilised after 190 years and was then used as a starting point for logging simulations. Selective logging regimes were run for both species for 300 years, with 100 repetitions of each simulation. Table 2 shows all management parameters used in simulations of D. odorata and J. copaia.
Table 2. Forest management parameters used for D. odorata and J. copaia Eco-gene simulations.
| Scenario | First year | Final Year | MCD (cm) | Cycle (years) | Intensity (%) |
|---|---|---|---|---|---|
| D. odorata | |||||
| 1 | 5 | 305 | 50 | 30 | 10, 30, 50, 70, 90 |
| 2 | 5 | 305 | 50 | 60 | 10, 30, 50, 70, 90 |
| 3 | 5 | 305 | 50 | 100 | 10, 30, 50, 70, 90 |
| 4 | 5 | 305 | 100 | 30 | 10, 30, 50, 70, 90 |
| 5 | 5 | 305 | 100 | 60 | 10, 30, 50, 70, 90 |
| 6 | 5 | 305 | 100 | 100 | 10, 30, 50, 70, 90 |
| 7 | 5 | 305 | 50 | No | 10, 30, 50, 70, 90 |
| 8 | 5 | 305 | 100 | No | 10, 30, 50, 70, 90 |
| J. copaia | |||||
| 1 | 5 | 305 | 50 | 30 | 10, 30, 50, 70, 90 |
| 2 | 5 | 305 | 40 | 30 | 100 |
| 3 | 5 | 305 | 30 | 30 | 100 |
| 4 | 5 | 305 | 50 | No | 10,30,50,70,90 |
Abbreviation: MCD, minimum cutting diameter at breast height. First year of logging; final year of logging; logging cycle and harvest intensity.
Data analysis
The results of the simulations were analysed for demographic (number of trees, number trees >10 cm d.b.h., number of reproductive trees, basal area) and genetic parameters (number of alleles, effective number of alleles, expected and observed heterozygosity, fixation index, Gregorius genetic distance, number of single locus genotypes; see Sebbenn et al., 2008). Data for each year were extracted as the mean values of 100 repetitions. Owing to limitations of the model, we treated D. odorata as a diploid species. Tetraploid species will be less sensitive than diploid species to the impacts of logging on genetic parameters. For example, observed heterozygosity requires more inbreeding to reach homozygosity and as such the analysis may overestimate the impacts of logging on genetic parameters of D. odorata.
For analysis of logging impacts, all parameters of a particular scenario were compared with the same parameter and year in the control scenario. Impacts of logging on demographic parameters were measured in three different ways for each scenario: (1) impact after the first logging event; (2) recovery of the parameter after a logging cycle; (3) minimum and maximum impact of logging after 300 years of forest management. The minimum was the impact 1 year before the next logging event and the maximum was the impact 1 year after the last logging event. Impacts of logging on genetic parameters were assessed after the first logging event and after 300 years of forest management. For a short-term validation, we performed comparisons of genetic data from one logging event (pre- and post-logging data) for D. odorata and J. copaia (Vinson, 2009) with simulated data from the Ecogene model, comparing number of alleles, observed and expected heterozygosity and fixation index.
Results
Control simulations
As both the D. odorata and J. copaia study populations were considered to be at equilibrium, no significant changes in either demographic or genetic parameters were expected over the 300 years without logging (control scenario) Eco-gene simulation. End results for demographic parameters in the D. odorata control showed similar values to the initial data. The input population had a basal area of 0.0765 m2 per ha, 250 trees >10 cm d.b.h. and 177 reproductive size trees, whereas after 300 years the control scenario had a basal area of 0.0754 m2 per ha, 260 trees >10 cm d.b.h. and 170 reproductive size trees.
End results for basal area of the J. copaia control were the same as the input (field data based), with 0.156 m2 per ha for the field population and 0.157 m2 per ha after 300 years of the control scenario. However, the number of trees (both >10 cm d.b.h. and reproductive) was lower in simulations; the control scenario having a stable population with 209 trees >10 cm d.b.h. compared with 256 trees in the field; and 133 reproductive size trees (>25 cm d.b.h.) compared with 155 such trees (>25 cm d.b.h.) in the field. Results for genetic parameters from the control scenario simulations were similar for both species, with the number of alleles, effective alleles, single locus genotypes, and expected and observed heterozygosities, all declining slightly during the simulation.
Length of recovery
A scenario simulating one J. copaia logging event at 50 cm MCD was used to estimate the recovery time of the population after logging. For the parameters basal area, number of trees and number of reproductive trees the recovery times were 23–76, 20–37 and 26–39 years respectively (10–90% removal of trees, >50 cm MCD).
As D. odorata has much slower growth rates, two MCD scenarios were used to assess recovery times of the population after logging. The first scenario (50 cm MCD) returned to the control scenario level over 190–275, 160–275, 151–269 years for basal area, number of trees and number of reproductive trees with 10–90% harvesting intensities. The second scenario (100 cm MCD) showed quicker recovery, for example, basal area recovered in 10, 102 and 185 years for 10%, 30% and 90% harvesting intensities, respectively.
J. copaia logging scenarios
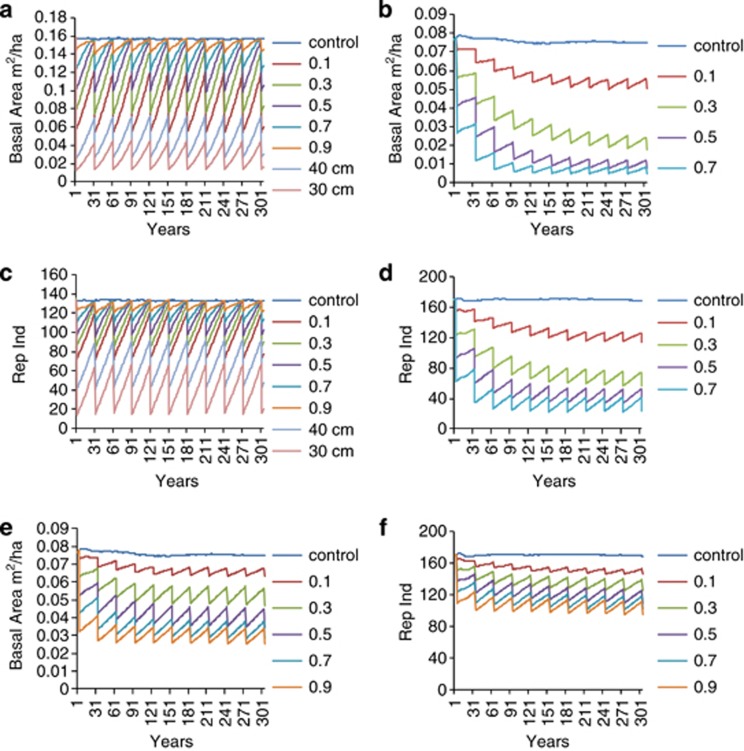
The Brazilian management scenario, with a 50-cm MCD and 30-year cycles, had no significant impact on J. copaia. Results with 70% or less harvesting intensity showed that demographic parameters recovered to the same level (±10%) as the control population within the first 30-year cycle and after 300 years of the Brazilian forest management regime (10 × 30-year logging cycles, 50 cm MCD; Figures 1a and c, Table 3). Whereas removal of 90% of the trees >50 cm d.b.h. resulted in a 24% decline in basal area, it can be considered sustainable in the sense that although the population is reduced it recovers to the same reduced level at the end of each 30 year cycle, that is, the population is not in continuous decline, with each cut producing the same yield. However, scenarios using 30 and 40 cm MCD are not sustainable for J. copaia (Figures 1a and c, Table 3).
Figure 1.
Simulations of (a) basal area (c) number of reproductive size trees for J. copaia under logging using 50 cm MCD with 0.1–0.9 intensity harvesting, and 40 cm MCD and 30 cm MCD with 100% intensity harvesting. Simulations of (b) basal area, (d) number of reproductive size trees for D. odorata under logging using 50 cm MCD with 0.1–0.7 intensity harvesting (simulations crash at 0.9 harvesting intensity because of reproduction failure). Simulations of (e) basal area and (f) number of reproductive size trees for D. odorata under logging using 100 cm MCD with 0.1–0.9 intensity harvesting.
Table 3. Impact of logging after 10 cycles of logging (300 years, 30-year cycles) on demographic parameters in J. copaia.
| MCD (cm) | Logging intensity (%) |
Basal area (m2 ha−1) |
Number of trees (>10 cm d.b.h.) |
Reproductive trees (>25 cm d.b.h.) |
|||
|---|---|---|---|---|---|---|---|
| Min (%) | Max (%) | Min (%) | Max (%) | Min (%) | Max (%) | ||
| 50 | 10 | 0 | 9 | 0 | 13 | 1 | 9 |
| 30 | 1 | 22 | 0 | 16 | 0 | 17 | |
| 50 | 2 | 37 | 0 | 19 | 1 | 27 | |
| 70 | 10 | 51 | 1 | 22 | 3 | 36 | |
| 90 | 24 | 65 | 3 | 25 | 10 | 45 | |
| 40 | 100 | 54 | 83 | 10 | 32 | 31 | 68 |
| 30 | 100 | 71 | 91 | 16 | 39 | 50 | 88 |
Abbreviations: Logging intensity, % of trees removed above MCD; Max, maximum; MCD, minimum cutting diameter at breast height; Min, minimum. Maximum value calculated as the impact 1 year after the last logging, minimum value calculated as the impact 1 year before the last logging event.
Standard deviations of control population are 0.006 (4%), 11.05 (2.7%) and 6.11 (4.5%) for basal area, number of trees and number of reproductive trees, respectively.
Impact after the first logging for D. odorata
The impacts 1 year after the first logging event show that the 50 cm MCD scenario reduced basal area by up to 85% and up to 82% of the reproductive trees in the population (Table 4). Although none of the cutting cycle treatments (30, 60 or 100 years) allowed demographic parameters to recover to the values in the original population, different effects were observed when MCD was varied. Using 100 cm MCD, all harvest intensities (except 10%) had smaller impacts on demographic parameters and greater recovery compared with the same harvesting intensities with 50 cm MCD (Table 4). The loss of reproductive trees is higher in the 50 cm MCD scenario than in the 100 cm MCD scenario for a similar loss of basal area. For example, 50% harvesting intensity in the 50 cm MCD scenario gave a 48% loss in basal area and 46% decrease in the number of reproductive trees, whereas for 70% harvesting intensity with 100 cm MCD, there is a 45% loss in basal area but only a 27% decrease in the number of reproductive trees (Table 4).
Table 4. Recovery of demographic parameters after the first logging cycle for D. odorata.
| MCD (cm) | Logging intensity (%) | Logging cycle (years) |
Demographic parameters |
||
|---|---|---|---|---|---|
| Basal area m2 ha−1 (%) | Number of trees (>10 cm d.b.h.) (%) | Reproductive trees (>35 cm d.b.h.) (%) | |||
| 50 | 10 | 30 | 92 | 94 | 92 |
| 60 | 92 | 95 | 93 | ||
| 100 | 94 | 97 | 96 | ||
| 30 | 30 | 75 | 84 | 77 | |
| 60 | 79 | 88 | 82 | ||
| 100 | 84 | 92 | 88 | ||
| 50 | 30 | 59 | 75 | 62 | |
| 60 | 64 | 79 | 69 | ||
| 100 | 72 | 86 | 79 | ||
| 70 | 30 | 40 | 64 | 46 | |
| 60 | 47 | 71 | 56 | ||
| 100 | 58 | 79 | 69 | ||
| 90 | 30 | X | X | X | |
| 60 | X | X | X | ||
| 100 | 37 | 70 | 55 | ||
| 100 | 10 | 30 | 95 | 97 | 96 |
| 60 | 96 | 98 | 98 | ||
| 100 | 97 | 99 | 99 | ||
| 30 | 30 | 87 | 94 | 91 | |
| 60 | 91 | 96 | 95 | ||
| 100 | 96 | 98 | 99 | ||
| 50 | 30 | 76 | 90 | 86 | |
| 60 | 86 | 95 | 93 | ||
| 100 | 94 | 98 | 98 | ||
| 70 | 30 | 66 | 87 | 80 | |
| 60 | 79 | 93 | 90 | ||
| 100 | 91 | 97 | 97 | ||
| 90 | 30 | 53 | 82 | 72 | |
| 60 | 68 | 89 | 84 | ||
| 100 | 84 | 96 | 94 | ||
Abbreviations: d.b.h., diameter at breast height; MCD, minimum cutting diameter at breast height; logging intensity, % of trees removed above MCD; X, no result because of population collapse.
Standard deviations of control population are 0.001 (2%), 4.2 (1%) and 3.4 (2%) for 30-year cycle, are 0.002 (3%), 4.8 (1.8%) and 4.2 (2.5%) for 60-year cycle and are 0.003 (4%), 5.5 (2%) and 5.1 (3%) for 100-year cycle for basal area, number of individuals and number of reproductive individuals, respectively.
D. odorata simulations with MCD of 50 cm d.b.h.
After 300 years of the Brazilian forest management regime (30-year cycles) with logging intensities of 70, 50 and 30%, the D. odorata population is in serious decline, and with 90% harvesting intensity the population collapses. Most of the timber stock is removed in the first three cycles, after which little timber is extracted per cycle (<0.05 m2 per ha basal area, Table 5), with very few trees and reproductive trees in the remaining population (Figures 1b and d). In addition, there was low recovery per cycle, with the population failing to recover to original levels and even to any lower equilibrium. A 60-year cycle would obviously allow greater recovery than a 30-year cycle, but the results still show drastic declines in the number of reproductive trees and basal area. Scenarios with 100-year cycles do not return to the control population levels but do reach a lower equilibrium, meaning that overall there is not a continual decline. However, the final population after 300 years is very reduced, from 170 to only 24 reproductive trees, under the most intensive harvesting scenario.
Table 5. Impacts after 300 years of logging, on demographic parameters and basal area extracted of D. odorata.
| MCD (cm) | Logging intensity | Logging cycle (years) |
Demographic parameters |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
Basal area m2 ha−1 |
Number of trees (>10 cm d.b.h.) |
Reproductive trees (>35 cm d.b.h.) |
Basal area extracted | ||||||
| Max (%) | Min (%) | Max (%) | Min (%) | Max (%) | Min (%) | m2 ha−1 | |||
| 50 | 10 | 30 | 33 | 26 | 25 | 16 | 32 | 25 | 0.065 |
| 60 | 18 | 9 | 16 | 6 | 18 | 9 | 0.041 | ||
| 100 | 13 | 4 | 12 | 3 | 13 | 4 | 0.021 | ||
| 30 | 30 | 76 | 67 | 47 | 36 | 66 | 56 | 0.114 | |
| 60 | 51 | 32 | 34 | 18 | 46 | 27 | 0.097 | ||
| 100 | 37 | 13 | 26 | 7 | 34 | 11 | 0.054 | ||
| 50 | 30 | 90 | 84 | 56 | 45 | 80 | 69 | 0.115 | |
| 60 | 77 | 60 | 48 | 29 | 68 | 45 | 0.120 | ||
| 100 | 62 | 28 | 41 | 14 | 56 | 22 | 0.076 | ||
| 70 | 30 | 93 | 89 | 60 | 49 | 86 | 76 | 0.109 | |
| 60 | 89 | 76 | 56 | 37 | 80 | 57 | 0.120 | ||
| 100 | 82 | 51 | 52 | 23 | 73 | 35 | 0.076 | ||
| 90 | 30 | X | X | X | X | X | X | X | |
| 60 | X | X | X | X | X | X | X | ||
| 100 | 93 | 70 | 59 | 30 | 86 | 47 | 0.057 | ||
| 100 | 10 | 30 | 15 | 9 | 13 | 6 | 13 | 10 | 0.050 |
| 60 | 10 | 4 | 9 | 3 | 8 | 4 | 0.028 | ||
| 100 | 8 | 2 | 8 | 1 | 6 | 2 | 0.015 | ||
| 30 | 30 | 37 | 24 | 21 | 12 | 26 | 18 | 0.117 | |
| 60 | 25 | 8 | 15 | 4 | 16 | 6 | 0.081 | ||
| 100 | 20 | 1 | 13 | 1 | 13 | 1 | 0.043 | ||
| 50 | 30 | 54 | 39 | 27 | 17 | 36 | 25 | 0.142 | |
| 60 | 41 | 14 | 21 | 6 | 25 | 9 | 0.124 | ||
| 100 | 33 | 3 | 17 | 1 | 20 | 2 | 0.070 | ||
| 70 | 30 | 62 | 49 | 30 | 19 | 40 | 30 | 0.143 | |
| 60 | 53 | 23 | 25 | 8 | 32 | 13 | 0.148 | ||
| 100 | 47 | 6 | 23 | 2 | 29 | 3 | 0.094 | ||
| 90 | 30 | 66 | 55 | 33 | 22 | 44 | 34 | 0.140 | |
| 60 | 63 | 35 | 29 | 12 | 39 | 18 | 0.120 | ||
| 100 | 60 | 13 | 28 | 4 | 37 | 6 | 0.104 | ||
Abbreviations: d.b.h., diameter at breast height; logging intensity, percentage of trees removed above MCD; Max, maximum; MCD, minimum cutting diameter at breast height; Min, minimum; X, no result because of population collapse. Maximum calculated as the impact one year after the last logging and minimum as the impact one year before the last logging event.
Standard deviations of control population are 0.003 (4%), 5.6 (2%), 5.5 (3%) and 0.003 (4%) for basal area, number of individuals, number of reproductive individuals, and extracted basal area, respectively.
D. odorata simulations with MCD of 100 cm d.b.h.
Overall, 100 cm MCD scenarios are more sustainable than 50 cm MCD scenarios. In most 100 cm MCD scenarios, the population reaches an equilibrium, such that the post-logging population does not continue to decline (Figures 1e and f). In addition, there was no population collapse under any 100 cm MCD scenario.
With the 30-year cycles timber stock declined over the first four logging cycles (Figures 1e and f). After that, the population did not continue to decline, with equal amounts of timber removed per cycle. For 60-year cycles with up to 50% logging intensities and for 100-year cycles (except 90% harvesting intensity) there was recovery of basal area to within 10% of the control population (Table 5). The number of reproductive trees was more sensitive than the number of trees, only recovering to within 10% of the control population with 100-year (all harvesting intensities) and 60-year (50, 30 and 10% harvesting) cycles. The scenarios with the greatest impact were with a 30-year cycle and harvesting intensities greater than 50%, resulting in 25–34% reductions in the number of reproductive trees. However, these reductions were minor compared with the 50 cm MCD scenarios, where impacts varied from 25% (10% harvesting) to 76% (70% harvesting) reductions in reproductive tree numbers, and population collapse when 90% of the reproductive trees were cut. Furthermore, 300 years of logging under the 100 cm MCD scenario would have removed more timber with 30-year cycles than the 50 cm MCD scenario with 30-year cycles, except in the case of a 10% harvesting intensity (Table 5).
Genetic parameters
For both study species, the genetic parameters most sensitive to logging were the numbers of alleles and single genotypes (Table 6). The loss of alleles and genotypes led to an increase in genetic distance, as calculated between each population at the start and end of the simulations. For both study species, after one logging event there was no impact on heterozygosity and fixation index in the simulated population, in agreement with the real population (Table 6). The fixation index in the control population was maintained at equilibrium after logging under all logging scenarios for both species (Table 6), as might be expected for outcrossing species with effective pollen and seed dispersal.
Table 6. Impact of 300 years of logging on genetic parameters in J. copaia and D. odorata.
| MCD (cm) | Logging intensity (%) | Cycle (years) | N | A | Ae | He | Ho | f | Dis | NG |
|---|---|---|---|---|---|---|---|---|---|---|
| J. copaia | ||||||||||
| Control | 399 | 77 | 5.7 | 0.825 | 0.745 | 0.097 | 0.030 | 401 | ||
| 50 | 10 | 30 | 397 | 77 | 5.7 | 0.826 | 0.746 | 0.097 | 0.030 | 402 |
| 30 | 30 | 397 | 76 | 5.7 | 0.825 | 0.745 | 0.096 | 0.029 | 397 | |
| 50 | 30 | 398 | 75 | 5.6 | 0.821 | 0.740 | 0.099 | 0.029 | 392 | |
| 70 | 30 | 396 | 73 | 5.6 | 0.822 | 0.739 | 0.101 | 0.028 | 381 | |
| 90 | 30 | 387 | 71 | 5.5 | 0.818 | 0.739 | 0.096 | 0.027 | 364 | |
| 40 | 100 | 30 | 360 | 67 | 5.3 | 0.810 | 0.735 | 0.093 | 0.024 | 328 |
| 30 | 100 | 30 | 336 | 59 | 5.0 | 0.797 | 0.726 | 0.089 | 0.022 | 270 |
| D. odorata | ||||||||||
| Control | 0 | 347 | 101 | 6.8 | 0.852 | 0.752 | 0.118 | 0.060 | 483 | |
| 50 | 10 | 30 | 304 | 99 | 6.7 | 0.851 | 0.747 | 0.122 | 0.052 | 458 |
| 60 | 331 | 100 | 6.8 | 0.852 | 0.750 | 0.120 | 0.053 | 477 | ||
| 100 | 339 | 101 | 6.8 | 0.852 | 0.748 | 0.122 | 0.055 | 484 | ||
| 30 | 30 | 252 | 94 | 6.4 | 0.844 | 0.739 | 0.124 | 0.048 | 406 | |
| 60 | 301 | 98 | 6.7 | 0.850 | 0.746 | 0.122 | 0.052 | 450 | ||
| 100 | 328 | 99 | 6.8 | 0.852 | 0.750 | 0.120 | 0.055 | 470 | ||
| 50 | 30 | 229 | 90 | 6.2 | 0.838 | 0.735 | 0.123 | 0.048 | 369 | |
| 60 | 270 | 94 | 6.4 | 0.844 | 0.740 | 0.123 | 0.051 | 418 | ||
| 100 | 310 | 97 | 6.6 | 0.849 | 0.743 | 0.124 | 0.053 | 453 | ||
| 70 | 30 | 250 | 91 | 6.3 | 0.842 | 0.738 | 0.123 | 0.049 | 390 | |
| 60 | 250 | 91 | 6.3 | 0.842 | 0.738 | 0.123 | 0.049 | 390 | ||
| 100 | 287 | 94 | 6.5 | 0.847 | 0.739 | 0.127 | 0.051 | 424 | ||
| 90 | 100 | 268 | 89 | 6.4 | 0.844 | 0.741 | 0.122 | 0.048 | 396 | |
| 100 | 10 | 30 | 330 | 101 | 6.8 | 0.853 | 0.750 | 0.121 | 0.055 | 479 |
| 60 | 339 | 102 | 6.8 | 0.853 | 0.748 | 0.124 | 0.054 | 487 | ||
| 100 | 344 | 101 | 6.8 | 0.853 | 0.748 | 0.123 | 0.055 | 485 | ||
| 30 | 30 | 317 | 99 | 6.7 | 0.851 | 0.748 | 0.122 | 0.054 | 463 | |
| 60 | 336 | 100 | 6.8 | 0.853 | 0.747 | 0.124 | 0.054 | 478 | ||
| 100 | 344 | 101 | 6.8 | 0.853 | 0.749 | 0.122 | 0.056 | 485 | ||
| 50 | 30 | 303 | 98 | 6.7 | 0.851 | 0.747 | 0.122 | 0.052 | 456 | |
| 60 | 332 | 100 | 6.8 | 0.853 | 0.749 | 0.121 | 0.055 | 476 | ||
| 100 | 344 | 101 | 6.8 | 0.853 | 0.749 | 0.121 | 0.055 | 486 | ||
| 70 | 30 | 296 | 98 | 6.7 | 0.850 | 0.745 | 0.124 | 0.052 | 447 | |
| 60 | 326 | 100 | 6.8 | 0.852 | 0.748 | 0.122 | 0.055 | 471 | ||
| 100 | 342 | 100 | 6.8 | 0.853 | 0.749 | 0.122 | 0.056 | 479 | ||
| 90 | 30 | 289 | 97 | 6.7 | 0.850 | 0.748 | 0.121 | 0.050 | 441 | |
| 60 | 315 | 98 | 6.7 | 0.851 | 0.752 | 0.117 | 0.054 | 463 | ||
| 100 | 336 | 100 | 6.8 | 0.852 | 0.750 | 0.121 | 0.054 | 479 | ||
Abbreviations: A, number of alleles; Ae, number of effective alleles; Dis, Gregorius genetic distance; He, expected heterozygosity; Ho, observed heterozygosity; f, fixation Index; MCD, minimum cutting diameter at breast height; logging intensity, % of trees removed above MCD; N, number of individuals analysed; NG, number of single genotypes.
Calculated 1 year before next logging event. Genetic parameters: N, A, Ae, He, Ho, f, Dis, NG.
Certain discrepancies between genetic data obtained in the field and from simulations were observed. For J. copaia, where the field population was sufficiently large to be used directly for simulations, in the real population there was a loss of two alleles, whereas in the simulated population there was no loss of alleles but a two-allele standard deviation (50 cm MCD with 50% intensity harvesting). For D. odorata, the number of alleles decreased by 10 after the first logging of the real population, whereas only two alleles (s.d.=0.3) were lost from equivalent logging in the simulated population (50 cm MCD with 50% intensity harvesting; Table 6). These results may relate to how Eco-gene generates a population, as the Eco-gene Data Generation Engine uses the same alleles and allele frequencies from a small field population to create a larger population. Consequently, the number of trees with rare alleles is higher in the simulated population and the probability that all the trees with a rare allele will be removed by logging is lower.
The impacts on genetic parameters of both species after 300 years of different management scenarios are detailed in Table 6. Among all logging simulations the treatment with the greatest impact on D. odorata genetic parameters was 50 cm MCD with 100-year logging cycles and 90% harvesting intensity. This scenario showed losses of 13 alleles (11% decrease) and 138 genotypes (Table 6). A slight reduction in observed and expected heterozygosity and an increase in fixation index suggests an increase of homozygotes in the population. For J. copaia, under the Brazilian management regime (50 cm MCD), there were decreases of between 1 and 6 in the total number of alleles and of up to 66 in the number of genotypes (Table 6). The greatest impacts were under the 30 cm MCD scenario, with up to 23% reduction in the number of alleles, 13% reduction in the number of effective alleles and a 38% loss in single genotypes (Table 6).
Discussion
Control scenario
Both species had control populations that were stable over the simulation period for basal area and with the same value as the initial population, that is, the field data. This indicates that the ecological parameters used for running the model were not only realistic, but also appropriate to generate the age, size and structure of the study population. The only discrepancy between simulated and field data was for J. copaia, where the number of trees (>10 cm d.b.h. and reproductive) was lower in simulations than in the field. One explanation for the discrepancy relates to the Eco-gene model being based on populations with an ‘inverted J' demography. As trees grow, they move from one d.b.h. class to a larger d.b.h. class, but if the larger class has a higher density, then the model does not create new trees to match the target value.
For both species, most of the genetic parameters declined during simulation of the control population, possibly because the model treats the study area as a fragment with no gene flow (pollen or seeds) from outside the plot. However, paternity analysis showed an influx of alleles from outside the study area for both species because of pollen flow (Vinson, 2009). As a consequence, the impacts of logging may be overestimated for the genetic diversity parameters.
Demographic parameters
Simulations allowed quantification of the long-term impacts on population demography for both species over successive logging cycles following Brazilian logging regulations. Logging of trees obviously reduces population size and basal area, hence for both species, among the demographic parameters analysed, basal area was the most sensitive, followed by number of reproductive trees and number of trees. This is due to the influence of individual tree diameters on basal area: bigger trees have greater basal area and these are removed by logging. The number of reproductive trees was more sensitive to density changes than the number of trees, as the number of trees includes a much higher proportion of trees that are not logged (below 50 cm d.b.h.).
The ability of growth parameters to recover from such reductions is dependent on growth rates, which are species- and site-specific, giving great variation in the recovery of population size and basal area between logging cycles (Sebbenn et al., 2008). In general terms, comparing the simulation results for the two species, J. copaia had the highest standing basal area, as well as the largest total basal area removed, even in the first cut. Most importantly, J. copaia was demographically stable under most scenarios, including the Brazilian management scenario. These results can be explained by J. copaia's fast growth rate and maximum diameter for survivorship (110 cm), such that smaller trees showed rapid growth into larger diameter classes, with rapid population turnover. In addition, the low d.b.h. at which J. copaia trees become reproductive ensured that, even after logging, many reproductive trees remained. Furthermore, a high proportion of these trees flower annually, which coupled with high synchrony of flowering, efficient pollination and dispersers, means that seedling establishment was annual and sapling growth kept the population large, even after logging.
Although the simulations showed that the 30-year logging cycles adopted by the Brazilian government (Barreto et al., 1998) are sustainable for J. copaia, they do not support current regulations for D. odorata management. Thirty years after one logging, the D. odorata population was still in recovery, only reaching a basal area equivalent to the original forest 190–275 years after the first cut. These results may be overly optimistic, as a C14 study of tree age demonstrated that some trees in an Amazonian forest, including D. odorata, were ancient, requiring 1200 years to reach 120 cm d.b.h. (Chambers et al., 1998).
In fact, this study showed that all selective logging below 100 cm MCD was likely to be unsustainable for D. odorata. A 90% harvesting intensity led to a collapse of the population, whereas other harvesting intensities (70, 50, 30 and 10%) at 50 cm MCD removed most of the timber stock in the first three cycles, after which the cost of logging would be high for little timber. In stark contrast, D. odorata scenarios using 100 cm MCD and 30-, 60- and 100-year cycles were all demographically stable, although at different levels. The populations were stable for 30- and 60-year cycles, but did not recover to their original levels, whereas if left for 100 years, demographic parameters recovered to within 10% of the original population. In relation to the cost-benefit of logging, all 100 cm MCD scenarios (with the exception of 10% harvest intensity), removed more timber stock than the current Brazilian forest management regime. Of the 100 cm MCD scenarios, the 100-year logging cycles removed less timber than 30- and 60-year cycles. The 100 cm MCD and 30-year cycles should be recommended for D. odorata, as they are likely to be more appealing in terms of timber volume extracted and the simultaneous logging of a number of species, while also having relatively minor impacts on population demography, reproduction and genetic structure, allowing a more consistent recovery of the species.
Of the species included in the Dendrogene project, Symphonia globulifera presented a similar pattern to J. copaia; both being pioneer species that reproduce at low sizes (20 cm d.b.h.) produce large amounts of seed that colonize gaps and grow quickly to large size trees (Sebbenn et al., 2008). Such species appear able to sustain relatively high levels of selective logging. But for any pioneer tree species that starts reproducing with d.b.h. close to the MCD, most of a population will be removed and recovery will be difficult. Pioneer species have few juveniles, as they grow quickly to large size in good light conditions. With such species, there would be need of specific gap management and clearing for their growth after logging (Jennings et al., 2001).
D. odorata is a climax species, with an ‘inverted J' size class distribution where most individuals are juveniles, smaller than those that are commercially exploited. As for other climax species studied by the Dendrogene project (Hymenea courbaril, Manilkara huberi, Bagassa guainensis; Silva, 2005; Azevedo, 2007; Lacerda, 2007; Sebbenn et al., 2008), the Brazilian forest management regulations, as well as a range of other scenarios, are shown to be unsustainable. Although these species have many juveniles, they do not reach adult size quickly because of slow growth and high minimum reproductive size (d.b.h. >40 cm), which is very close to the current MCD limit. For H. courbaril and M. huberi, it took 120 and 150 years, respectively, to recover initial basal areas (Azevedo, 2007; Lacerda, 2007). Thus, there should be specific management of slow-growing species that reproduce only when very large. Once the minimum size at which a species is reproductive is known, management practices can be implemented that ensure the presence of sufficient trees greater than this size before the next cut (Jennings et al., 2001).
Unfortunately, the commercial vision of logging companies is restricted, as it is not in their interests to optimise logging cycles for sustained long-term timber production. Therefore, most timber stock is generally removed in the first cycle to maximise profits, with concomitant declines in the tree populations and other serious impacts on the forest. Although government is under pressure, decisive action must be taken to achieve sustainable forest management. Most forest management plans of other tropical countries are more restrictive (Silva et al., 1989), for example, French Guyana uses a 60-cm MCD and 65-year cycle. Based on simulations, others have also recommended more restrictive management practices, including increased MCD and longer cutting cycles (Schulze et al., 2005; van Gardingen et al., 2006; Sebbenn et al., 2008). Our results corroborate these recommendations, but for practical reasons, we suggest that logging cycles are not changed, but MCD is increased to 100 cm d.b.h. with avoidance of harvesting intensities ⩾90% for slow-growing species, especially species with large minimum reproductive d.b.h.
Genetic parameters
The removal of large trees may lead to direct losses of genetic diversity thereby increasing the likelihood of bottlenecks, genetic drift, dysgenic selection and reproductive issues (for example, inbreeding depression; Jennings et al., 2001). Among the genetic parameters, number of alleles and number of single genotypes were the most sensitive to logging, whereas number of effective alleles, genetic distance, fixation index, observed and expected heterozygosity were the least sensitive. Overall, the simulations showed little impact on genetic parameters for J. copaia, where the impact of logging on the number of reproductive trees was small, and hence, there was no impact on the number of alleles and number of genotypes. For D. odorata, the scenario with the greatest impact on genetic parameters was the current Brazilian forest management regime, with slight reductions in the number of rare alleles, single genotypes, expected and observed heterozygosity, and an increase in fixation index.
In general, the small impacts on genetic parameters can be explained by the long-lived nature of the trees species. As their life expectancy is around 300 years, many trees present at the beginning of the simulation will remain at the end of the simulation, leaving a ‘genetic footprint' within the population and limiting the extent to which changes in genetic parameters occur. Detection of increases in homozygosity and fixation index may require many more generations of trees to be simulated. The low sensitivity of heterozygosity to human impacts has been seen in other studies of diploid (for example, Cloutier et al., 2007; Lacerda et al., 2008) and tetraploid neotropical tree species (Hanson et al., 2008; Ng et al., 2009), whereas mating system parameters appear to be more sensitive (Ng et al., 2009; Azevedo et al., 2007). The inclusion of parameters related to mating systems in the model may offer a more sensitive tool for understanding the consequences of reductions in effective population size from logging and for the viability of tree populations.
Although the effects on genetic diversity parameters were small, because the current MCD is close to the minimum reproductive d.b.h. of D. odorata, its effective population size is dramatically reduced. Small effective population size can limit reproduction and mating system processes causing reductions or biases in the gene pool associated with bottleneck effects. As population size reduces, the number of possible mates is reduced, with increases in inbreeding anticipated, especially in species with asynchronously flowering populations (for example, D. odorata, Vinson, 2009) or short pollen and seed dispersal (for example, Manilkara huberi; Azevedo et al., 2007). These factors can directly affect the fitness of seeds and consequently population regeneration during logging cycles. The extent to which such impacts occur will, in part, be affected by the size of forest under management and whether such genetic bottlenecks and associated inbreeding are reduced or avoided by gene flow from surrounding areas. Forest fragmentation is likely to increase these impacts.
To design sustainable forest management plans it is important to carry out studies that compare how the genetic parameters of contrasting species respond to selective logging. Although D. odorata is an autotetraploid species, there are many tropical tree species with similar life history traits that are diploid, and which are therefore likely to be affected in a similar way by selective logging. Eco-gene may therefore not only allow us to explore how the population of D. odorata may respond to selective logging but also to extrapolate these results to similar species.
Conclusions
Simulations according to Brazilian management regulations showed little impact on genetic parameters for J. copaia and only slight impacts for D. odorata, that is, slight reductions in numbers of rare alleles and single genotypes, expected and observed heterozygosity and an increase in fixation index. These two tree species are long lived, and so large changes in genetic parameters may require many generations and significantly longer than 300 years. The extent and speed of these impacts will also depend on the size of the logged area and whether there is external gene flow into the logged area.
In this study, demographic parameters were more sensitive to selective logging than genetic parameters, with J. copaia much more resilient than D. odorata. Brazilian management regulations are sustainable for J. copaia, both for timber extraction and conservation of the species. However, D. odorata cannot cope with current regulations; simulations predict population collapse or a drastic decrease in population size, because of the slow growth and large proportion of reproductive trees removed in each logging event. D. odorata trees reach up to 200 cm d.b.h., but MCD is 50 cm d.b.h., with trees only reproductive from about 39 cm d.b.h. Raising the MCD to 100 cm d.b.h. in combination with 30-year logging cycles and harvesting intensities below 90%, both remove more timber volume in the long term and at the same time generate minor impacts on population demography, reproduction and genetic structure. This management practice could potentially allow more consistent recovery of the species over repeated logging cycles.
Crucially for forest management practice, our results show that the study species respond differently, both demographically and genetically, to the logging scenarios. Truly sustainable forest management will require regulations that are specific to ecological groups, and take into account the varying life-history traits of different timber species. In this way and with implementation of reduced impact logging practices, sustainable forest management could be possible for some tropical forest tree species.
Data archiving
Data available from the Dryad Digital Repository: doi:10.5061/dryad.6jh51.
Acknowledgments
This work was supported by the Dendrogene Project I (2000–2004) executed by Embrapa Amazônia Oriental and their partners in the bilateral cooperation between Brazil and United Kingdom through the Brazilian Cooperation Agency (ABC) and the Department for International Development (DFID); and Dendrogene Project II (2005 to present) executed by Embrapa Amazônia Oriental and their partners and supported by grants from FINEP/MCT/CNPq in Brazil. We also thank Ian Thompson, Milton Kanashiro and Lucia Wadt for project coordination.CCV received a fellowship from Clarendon Programme (University of Oxford), Beca Program-IEB/Fundação Moore (Scholarship No. B.2005/01/BDE/011) and Program Alβan, the European Union Program of High Level Scholarships for Latin America (Scholarship No. E05D054628BR). DHB was supported by the Seedsource project EC (FP6–2002-INCO-DEV-1).
The authors declare no conflict of interest.
References
- Alder D, Silva JNM. (2000). An empirical cohort model for management of Terra Firme forests in the Brazilian Amazon. For Ecol Manage 130: 141–157. [Google Scholar]
- Azevedo NCR, Kanashiro M, Ciampi AY, Grattapaglia D. (2007). Genetic structure and mating system of Manilkara huberi (Ducke) A. Chev., a heavily logged Amazonian timber species. J Hered 98: 646–654. [DOI] [PubMed] [Google Scholar]
- Azevedo VCR. (2007) Desenvolvimento e aplicações de microssatélites, análise de cpDNA e modelagem computacional para estudos da estrutura e dinâmica genética de maçaranduba—Manilkara huberi (Ducke) Chev. Sapotaceae. PhD thesis, Universidade de Brasília, Brasília.
- Barreto P, Amaral P, Vidal E, Uhl C. (1998). Costs and benefits of forest management for timber production in eastern Amazonia. For Ecol Manage 108: 9–26. [Google Scholar]
- Chambers JQ, Higuchi N, Schimel JP. (1998). Ancient trees in Amazonia. Nature 391: 135–136. [Google Scholar]
- Cloutier D, Kanashiro M, Ciampi AY, Schoen DJ. (2007). Impact of selective logging on inbreeding and gene dispersal in an Amazonian tree population of Carapa guianensis Aubl. Mol Ecol 16: 797–809. [DOI] [PubMed] [Google Scholar]
- Dauber E, Fredericksen TS, Pena-Claros M. (2005). Sustainability of timber harvesting in Bolivian tropical forests. For Ecol Manage 214: 294–304. [Google Scholar]
- De Graaf NR. (2000). Reduced impact logging as part of the domestication of neotropical rainforest. Int For Rev 2: 40–44. [Google Scholar]
- Degen B, Blanc L, Caron H, Maggia L, Kremer A, Gourlet-Fleury S. (2006). Impact of selective logging on genetic composition and demographic structure of four tropical tree species. Biol Conserv 131: 386–401. [Google Scholar]
- Degen B, Gregorius HR, Scholz F. (1996). ECO-GENE, a model for simulation studies on the spatial and temporal dynamics of genetic structures of tree populations. Silvae Genetica 45: 323–329. [Google Scholar]
- Degen B, Roubik D, Loveless MD. (2002). Impact of selective logging and forest fragmentation on the seed cohorts of an insect-pollinated tree: a simulation study. In: Degen B, Loveless MD, Kremer A (eds) Modeling and Experimental Research on Genetic Process in Tropical and Temperate Forest. EMBRAPA Amazônia Oriental: Belém, Brazil. pp 108–110. [Google Scholar]
- Fredericksen TS, Putz FE, Pattie P, Pariona W, Pena-Claros M. (2003). Sustainable forestry in Bolivia - Beyond planning logging. J For 101: 37–40. [Google Scholar]
- Gourlet-Fleury V, Favrichon S, Petronelli P. (2004). Consequences of silvicultural treatments on stand dynamics at Paracou. In: Gourlet-Fleury V, Guehl JM, Laroussinie O (eds). Lessons Drawn from Paracou, a Long-term Experimental Research Site in French Guiana. Elselvier: Paris. pp 254–280. [Google Scholar]
- Hanson TR, Brunsfeld SJ, Finegan B, Waits LP. (2008). Pollen dispersal and genetic structure of the tropical tree Dipteryx panamensis in a fragmented Costa Rican landscape. Mol Ecol 17: 2060–2073. [DOI] [PubMed] [Google Scholar]
- Jennings SB, Brown ND, Boshier DH, Whitmore TC, Lopes JDA. (2001). Ecology provides a pragmatic solution to the maintenance of genetic diversity in sustainably managed tropical rain forests. For Ecol Manage 154: 1–10. [Google Scholar]
- Kammesheidt L, Kohler P, Huth A. (2001). Sustainable timber harvesting in Venezuela: A modelling approach. J Appl Ecol 38: 756–770. [Google Scholar]
- Lacerda AEBd. (2007). Ecological and Genetic Impacts of Reduced-Impact Logging in the Brazilian Amazonian Forest: the Case of Hymenaea courbaril L. University of Reading. School of Human and Environmental Sciences: Reading.
- Lacerda AE, Kanashiro M, Sebbenn AM. (2008). Effects of Reduced Impact Logging on genetic diversity and spatial genetic structure of a Hymenaea courbaril population in the Brazilian Amazon Forest. For Ecol and Manage 255: 1034–1043. [Google Scholar]
- Maués MM. (2006). Estratégias Reprodutivas de Espécies arbóreas e a Sua Importância Para o Manejo e Conservação Florestal: Floresta Nacional do Tapajós (Belterra-PA). Unpublished PhD thesis, Universidade de Brasília: Brazil. [Google Scholar]
- Maués MM, Oliveira PEAMD, Kanashiro M. (2008). Pollination biology in Jacaranda copaia (Aubl.) D. Don. (Bignoniaceae) at the ‘Floresta Nacional do Tapajós', Central Amazon, Brazil. Revista Brasileira de Botânica 31: 517–527. [Google Scholar]
- Muller-Landau HC, Condit RS, Harms KE, Marks CO, Thomas SC, Bunyavejchewin S et al. (2006). Comparing tropical forest tree size distributions with the predictions of metabolic ecology and equilibrium models. Ecol Lett 9: 589–602. [DOI] [PubMed] [Google Scholar]
- Ng KKS, Lee SL, Ueno S. (2009). Impact of selective logging on genetic diversity of two tropical tree species with contrasting breeding systems using direct comparison and simulation methods. For Ecol Manage 257: 107–116. [Google Scholar]
- Pena-Claros M, Fredericksen TS, Alarcon A, Blate GM, Choque U, Leano C et al. (2008). Beyond reduced-impact logging: silvicultural treatments to increase growth rates of tropical trees. For Ecol Manage 256: 1458–1467. [Google Scholar]
- Pinard MA, Cropper WP. (2000). Simulated effects of logging on carbon storage in dipterocarp forest. J Appl Ecol 37: 267–283. [Google Scholar]
- Putz FE, Sist P, Fredericksen T, Dykstra D. (2008). Reduced-impact logging: challenges and opportunities. For Ecol Manage 256: 1427–1433. [Google Scholar]
- Schulze M, Vidal E, Grogan J, Zeweede J, Zarim D. (2005). Madeiras nobres em perigo: As melhores práticas de manejo atuais não sustentarão a produção de madeira nas florestas da Amazônia. Ciência Hoje 214: 66–69. [Google Scholar]
- Sebbenn AM, Degen B, Azevedo VCR, Silva MB, de Lacerda AEB, Ciampi AY et al. (2008). Modelling the long-term impacts of selective logging on genetic diversity and demographic structure of four tropical tree species in the Amazon forest. For Ecol Manage 254: 335–349. [Google Scholar]
- Silva JNM. (1989). The behaviour of the tropical rain forest of the Brazilian Amazon after logging. Unpublished D.Phil. Thesis, Oxford Forestry Institute, University of Oxford: Oxford, UK. [Google Scholar]
- Silva MB. (2005). Características ecológicas e genéticas de Bagassa guianensis Aubl. (Moraceae): elementos para o manejo florestal. Unpublished PhD Thesis. Universidade Federal do Pará: Brazil. [Google Scholar]
- Sist P, Ferreira FN. (2007). Sustainability of reduced-impact logging in the Eastern Amazon. For Ecol Manage 243: 199–209. [Google Scholar]
- Sist P, Fimbel R, Sheil D, Nasi R, Chevallier MH. (2003). Towards sustainable management of mixed dipterocarp forests of South-east Asia: moving beyond minimum diameter cutting limits. Environ Conserv 30: 364–374. [Google Scholar]
- Sist P, Garcia-Fernandez C, Fredericksen TS. (2008). Moving beyond reduced-impact logging towards a more holistic management of tropical forests. For Ecol Manage 256: VII–IX. [Google Scholar]
- Valle D, Schulze M, Vidal E, Grogan J, Sales M. (2006). Identifying bias in stand-level growth and yield estimations: a case study in eastern Brazilian Amazonia. For Ecol Manage 236: 127–135. [Google Scholar]
- van Gardingen PR, McLeish MJ, Phillips PD, Fadilah D, Tyrie G, Yasman I. (2003). Financial and ecological analysis of management options for logged-over Dipterocarp forests in Indonesian Borneo. For Ecol Manage 183: 1–29. [Google Scholar]
- van Gardingen PR, Valle D, Thompson I. (2006). Evaluation of yield regulation options for primary forest in Tapajos National Forest, Brazil. For Ecol Manage 231: 184–195. [Google Scholar]
- Vinson CC. (2009) Impact of Selective Logging on Inbreeding and Gene Flow in Two Amazonian Timber Species with Contrasting Ecological and Reproductive Characteristics. Unpublished D.Phil. Thesis, University of Oxford: Oxford, UK. [DOI] [PubMed] [Google Scholar]
- Vinson CC, Ribeiro DO, Harris SA, Sampaio I, Ciampi AY. (2009). Isolation of polymorphic microsatellite markers for the tetraploid Dipteryx odorata, an intensely exploited Amazonian tree species. Mol Ecol Resour 9: 1542–1544. [DOI] [PubMed] [Google Scholar]
- Vinson CC, Sampaio I, Ciampi A. (2008). Eight variable microsatellite loci for a Neotropical tree, Jacaranda copaia (Aubl.) D.Don (Bignoniaceae). Mol Ecol Resour 8: 1288–1290. [DOI] [PubMed] [Google Scholar]