Abstract
Background:
Oxidative stress has been a frequent finding in epileptic patients receiving antiepileptic drugs (AEDs). In this study, the influence of Vitamin E on the antiseizure activity and redox state of patients treated with carbamazepine, sodium valproate, and levetiracetam has been investigated.
Materials and Methods:
This double-blind, placebo-controlled trial was carried out on 65 epileptic patients with chronic antiepileptic intake. The subjects received 400 IU/day of Vitamin E or placebo for 6 months. Seizure frequency, electroencephalogram (EEG), and redox state markers were measured monthly through the study.
Results:
Total antioxidant capacity, catalase and glutathione were significantly higher in Vitamin E received group compared with controls (P < 0.05) whereas malodialdehyde levels did not differ between two groups (P < 0.07). Vitamin E administration also caused a significant decrease in the frequency of seizures (P < 0.001) and improved EEG findings (P = 0.001). Of 32 patients in case group, the positive EEG decreased in 16 patients (50%) whereas among 33 patients in control group only 4 patients (12.1%) showed decreased positive EEG.
Conclusion:
The results of this preliminary study indicate that coadministration of antioxidant Vitamin E with AEDs improves seizure control and reduces oxidative stress.
Keywords: Carbamazpin, electroencephalogram, epilepsy, levetiracetam, oxidative stress, sodium valproate, Vitamin E
INTRODUCTION
Epilepsy is a highly prevalent important neurological disease in the world. At least 50 million people worldwide are affected by the disease and nearly 100 million people have experienced seizure at least once in their lives. The disease creates psychological, physical, social, and economic consequences. Its prevalence is 5.8/1000 in developed countries and 10.3/1000 people in developing countries.[1]
The most commonly used treatments for epilepsy are antiepileptic drugs (AEDs). The most common drug used to treat epilepsy is valproate (VPA) sodium, carbamazepine, phenytoin, phenobarbital, and lamotrigine and currently levetiracetam. Usually a combination of drugs is used to treat epilepsy. Choosing any of these drugs depends on the type of seizure and the involved part in the brain. One of the functions of carbamazepine and sodium VPA and levetiracetam is through the impact on the course of oxidative stress.[2,3,4,5]
VPA can also reduce the concentration of the mitochondrial coenzyme A which is produced due to the conversion of the VPA to valproyl-CoA in the presence of adenosine triphosphate (ATP) and coenzyme A. This may affect the oxidation of fatty acids in the mitochondria and impaired ATP synthesis. Fatty acid oxidation by VPA can also be caused by the effect of this drug on carnitine.[1,6]
Oxidative stress is a mitochondrial dysfunction and an imbalance between pro-oxidant and antioxidant and is involved in many pathological and physiological processes. Brain cells are sensitive to reactive oxygen types that are produced during the oxidation.[7]
Brain cells are sensitive to reactive oxygen species. Oxidative stress can cause irreversible damage to biological molecules such as proteins, lipids, carbohydrates, and the DNA. Oxidative stress is involved in many neurodegenerative disorders such as epilepsy.[4,5,6,7,8,9,10,11]
Vitamin E (α-tocopherol) is a lipophilic alcohol and its food source is the root of wheat and vegetable oils. The most important part of this substance is the α part because it forms 90% of the tocopherol composition of animal tissues. Many physiological functions have been considered for this substance including stabilization of membrane, acting as enzyme inhibitor and the multiplier of the effect of Vitamin A2. This substance has the ability to prevent the negative effects of lipid peroxidation in the brain tissue because it can absorb free radicals of oxygen. Many studies have shown the effect of Vitamin E on the treatment of epilepsy. For example, in a study, this substance was used as adjunctive therapy in the treatment of epilepsy in children whose seizures were not well controlled, resulting in significant improvement. It has no serious side effects and risk of toxicity. This causes the substance to be considered in the treatment of epilepsy.[6,12,13]
Many studies have shown an increase in oxidative stress in epilepsy and have stated that free radicals act as a pathogen in the disease.[4,5,8]
Other studies have shown the effect of antiepileptic on enhancing free oxygen species.[5,6,7,8,9,10,11]
In 2007, a study was conducted in children with epilepsy, and it was found that adding Vitamin E to AEDs reduces their seizures.[5]
In another study conducted by Kovalenko et al., it was found that adding Vitamin E to antiepileptic reduces plasma levels of lipid peroxidation and decreases the frequency of seizures in these patients.[14]
Among the studies, the lack of a study showing the effect of Vitamin E as an antioxidant on the reduction of oxidative stress created both by anticonvulsants and the epilacy in adults was felt which could show this effect using both oxidative and antioxidative findings of blood together with electroencephalogram (EEG) findings of patients. It also seems that there is no study about the effects of Vitamin E on EEG findings.
Due to the abovementioned cases and because of high frequency of epilepsy in Iran[15,16] and in the world,[1] which cause injuries to the social, occupational, and other fields of life, research into finding ways to help the treatment of this disease is a big step towards helping these patients.
As discussed, an important damaging factor causing seizures is the destruction of neurons with oxidant antioxidant system imbalance and increase of active oxygen species caused by oxidative stress.[4,10,12] As a result, it was decided to make use of a substance in order to reduce the effect of oxidants, consequently increasing the efficacy of anticonvulsants so that the patients could benefit most from their treatment. Vitamin E is a natural antioxidant free of important side effects and it can easily be used by patients as an adjunctive therapy.[6,12,13] According to this hypothesis, a study was designed to investigate the effect of Vitamin E on improving the effectiveness of AEDs in epilepsy control.
MATERIALS AND METHODS
This is a double-blind, controlled clinical trial study on patients with intractable epilepsy treated by AEDs referred to Kashani Hospital and private epilepsy clinics in Isfahan. The sampling method was simple non-random, and the experiment and control groups were assigned randomly.
Inclusion criteria were as below:
Age 20–50 years
Signing a consent form to participate in the study
Diagnosis of refractory epilepsy) The patients who meet this definition subsequently achieve prolonged (6 months or more) periods of seizure remission with at least two AEDs): (1) At least 2 seizures per month (2) taking at least two standard anticonvulsant medications in the past 6 months
The ability of family or friends to count the number of seizures in epileptic patients during the determined period
Patients taking carbamazepine, sodium VPA, levetiracetam, or a combination of them
Not taking Vitamin E 6 months before the study
Lack of developing diseases such as liver and kidney failure.
Moreover, the patients with lack of consent to continue with the study were excluded.
Initially, 66 epileptic patients of all types were assessed by the executive team considering their inclusion and exclusion criteria. Their basic and demographic information, the frequency of their seizures and EEG findings (interictal epileptiform sharp wave) were gathered in a checklist. Then, sampling was performed on their venous blood to measure biomarkers produced during the oxidative process involving total antioxidant capacity, catalase, and malondialdehyd. Plasma antioxidant capacity was measured using Benzie IF method.[17] Briefly, in this method, the recovery power of ferric to ferrous ions at low pH produces a down more color complex of pyridyl triazine which can be measured by absorption changes at 593 nm-wavelengths.
Measurement of catalase was performed based on Góth[18] spectrophotometry method. In this method, serum were incubated with the substrate hydrogen peroxide and then the enzymatic reaction was stopped by the addition of ammonium molybdate and finally the light absorption of molybdate yellow and hydrogen peroxide was measured at 405 nm-wavelength.
Serum malondialdehyde levels were measured using the method introduced by Satoh.[19] In this method, serum proteins were precipitated by trichloroacetic acid and then the supernatant liquid was combined with a solution of thiobarbituric acid reagent and was boiled for 30 min. The resulting color combination is extracted using n-butanol and its concentration is measured at 532 nm-wavelengths.
After receiving their consent for participating in the study, the patients were randomly divided into two groups of control and intervention. The intervention group (n = 33) were given daily supplements of 400 IU Vitamin E. The control group (n = 33) received placebo pills identical in appearance to Vitamin E. The prescribing physician did not know about the type of drug, and the drug was determined by codes A and B. At the beginning of the study all patients (controls and interventions) underwent EEG.
Patients were visited on monthly basis to check the frequency of seizures and at the end of 6 months EEG changes (decreased or increased in interictal epileptiform sharp wave) and biochemical markers were measured. The frequency of seizures were evaluated by enumeration and comparison of spike and sharp wave and interictal epileptiform sharp wave during the 6 months period of this study and the number of seizures per patient were counted and compared to previous waves were.
Interpretation of EEGs were randomly carried out by each of the two neurologist physicians with prolonged experience in management of epilepsy.
EEGs equipment Model NIHON KOHDEN with longitudinal bipolar and ear referential montage.
Survey of the role of many other clinical criteria, especially brain pathology, were beyond the scope of our preliminary study. The designed limited number of cases does not permit us to further categorize the patients according to their brain pathology.
The obtained data were entered into SPSS software version 17.01 and were analyzed using Chi-square test and independent T-test.
RESULTS
Figure 1 shows profile of the study, of 77 reviewed patients, 7 patients did not enter to the study (3 patients were not eligible and 4 patients refused informed consent). About 70 patients were eligible and randomly assigned in to two intervention groups. During following period 3 patients in case group and 2 patients in control group did not desire to continuo and excluded, finally, 32 cases and 33 controls completed the study and analyzed. No signs of toxicity, drugs adverse effects or worsening of patient's clinical status were observed during this study.
Figure 1.
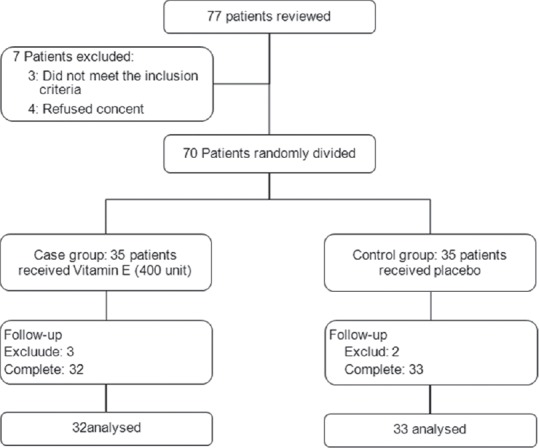
Patients who entered to the study, divided into the study groups and analyzed
The mean of age in studied patients were 28.7 ± 7.2 years old. Of studied patients, 38 (58.5%) were male and 27 (41.5%) were female. Table 1 shows baseline characteristics of studied patients. No significant differences were noted between case and control groups for age, sex, AEDs, and seizures types (P > 0.05). Most of the cases used carbamazepine + sodium VPA as AEDs and in controls sodium VPA + levetiracetam was the most used AEDs. Focal seizure was more frequent in control group and generalized seizure was more frequent in case group.
Table 1.
Baseline characteristics in studied patients
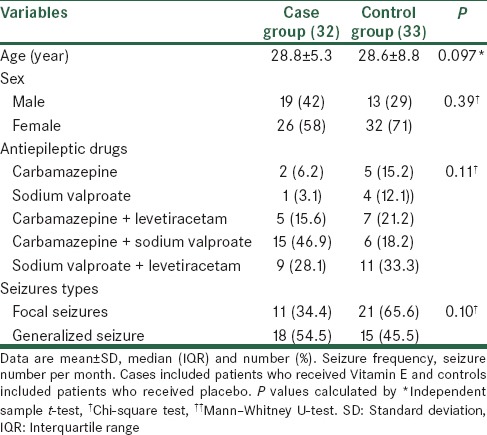
Table 2 shows the result of comparison of the mean of antioxidant parameters and seizure frequency, before and after treatment between study groups. As shown, total antioxidant, catalase, and malondialdehyde before treatment in control group were significantly higher than in case group (P < 0.05). Glutathione before treatment in both groups were similar with no significant differences (P > 0.05). After treatment total antioxidant capacity, catalase and glutathione in both groups were similar and no significant differences were noted (P > 0.05). Malondialdehyde in control group was significantly higher than in case group (P = 0.001). After treatment, in case group, total antioxidant capacity, catalase and glutathione were significantly increased in compare to before treatment (P < 0.05), and malondialdehyde decreased but was not statistically significant (P = 0.1). In control group, the mean of studied parameters after treatment were not significantly different compare to before treatment (P > 0.05). Furthermore, the mean of increase in cases was significantly higher than in controls for total antioxidant capacity, catalase, and glutathione (P < 0.05), but for malondialdehyde was not significantly different between groups (P = 0.07). Seizure frequency before treatment was similar between groups (P = 0.89) but after treatment the frequency of seizure in case group decreased and was significantly lower than control group (P < 0.0001). After treatment, in comparison to before treatment, the frequency of seizure in both cases and controls was significantly different (P < 0.05).
Table 2.
Antioxidant parameters and seizure frequency in studied patients
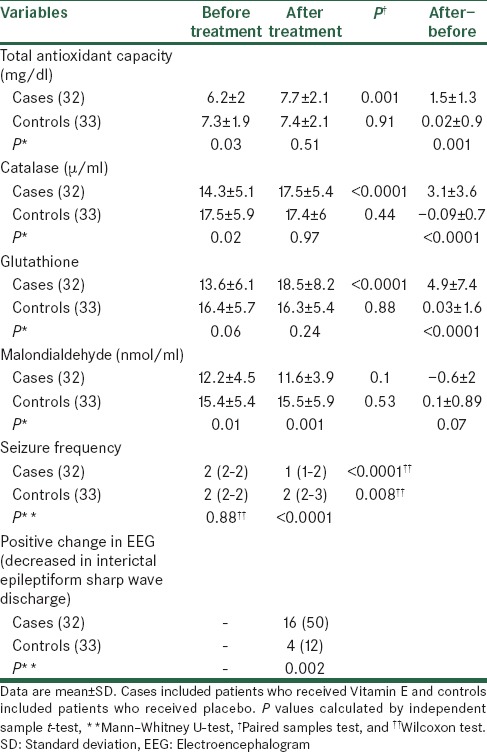
Figure 2 shows the comparison of the trend of chances in antioxidant parameters after treatment in comparison to before treatment between case and control groups. As shown, chances in trend of total antioxidant capacity, catalase, and glutathione after treatment in compare to before treatment were not statistically significant between groups (P > 0.05), but chance in trend of malondialdehyde after treatment in compare to before treatment between groups was statistically significant (P = 0.003).
Figure 2.
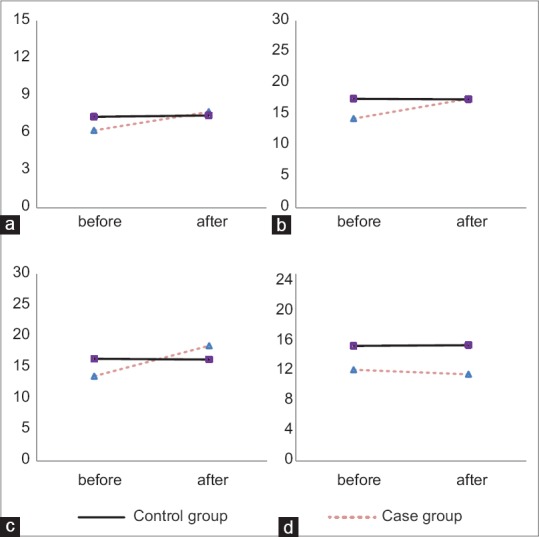
Comparison of antioxidant parameters between study groups by paired samples test. Case group included patients who received Vitamin E and control group included patients who received placebo. The difference of the trend of parameters were not significantly different between study groups for total antioxidant capacity (a, P = 0.44), catalase (b, P = 0.25), glutathione (c, P = 0.79), and was significantly different for malondialdehyde (d, P = 0.003)
Decreased in the positive EEG findings in case group were observed more than control group. Of 32 patients in case group 16 patients (50%) had decreased in the positive EEG findings and 16 patients (50%) did not have decreased in the positive EEG findings whereas among 33 patients in control group only 4 patients (12.1%) had decreased in the positive EEG findings and 29 patients (87.9%) did not have decreased in the positive EEG findings [P = 0.001, Table 2].
DISCUSSION
In patients with epilepsy, the deficiency of Vitamin E has been reported, this deficiency has been attributed to antiepileptic therapy, and however the antiepileptic effect of Vitamin E is contrary.[20,21] This randomized controlled trial was aimed to assess the effect of Vitamin E on the seizure frequency, and biochemical parameters and EEG finding in added to antiepileptic therapy in refractory epileptic patients. Our findings show that total antioxidant capacity, catalase, and glutathione significantly increased in epileptic patients after adding Vitamin E to their antiepileptic therapy in compare placebo. In addition, the frequency of seizure significantly decreased in patients who received Vitamin E than placebo group. Another important of our finding was that decreased in positive change in EEG findings in case group was significantly more than control group. Thus, Vitamin E may be suggested as an adjunctive antiepileptic therapy with partial success.
Previously, it is show that in epileptic patients lipid peroxidation, glutathione peroxidase, as parameters of oxidative stress were significantly higher when compared to controls, either levels of Vitamin C, E, and A, as antioxidant substances were significantly lower in epileptics when compared to controls, and suggesting that free radicals may be implicated in epilepsy.[22,23] One of the lipophilic antioxidant that is able to penetrate the blood-brain barrier and accumulate at high concentration in the brain is Vitamin E.[24] Therefore, suggesting that in suppressing seizures and neuronal damage, Vitamin E supplementation can be helpful. In one animal study, effect of Vitamin E in the standard animal seizure models has been assessed and authors concluded that Vitamin E has an anticonvulsant effect in animal seizure models.[25] In a double-blind, cross-over trial, by Raju et al. Vitamin E as add-on therapy compared to placebo in patients with uncontrolled epilepsy. After two treatment phases of Vitamin E or placebo with cross-over to the second phase, authors reported that change in seizure frequency observed with Vitamin E as compared with placebo was not significant between two treatments.[26] Other studies show that treatments with Vitamin E have had beneficial effects on seizure activity and neurodegeneration induced by pentylentetrazol[27] or pilocarpine.[28] Moreover, Vitamin E attenuated lipid peroxidation and increased catalase activity after pilocarpine-induced seizure[29,30] and reduced blood-brain barrier disruption after pentylentetrazol-induced epileptic seizure.[27] In contrast, some studies reported that Vitamin E can not to reduce seizure activity induced by amygdala kindling or KA.[31] Similar to some studies and in contrast to others in the present study, Vitamin E as an add-on therapy for epileptic patients, who received multi- or single-drug therapy, can be effective to significantly increased total antioxidant capacity, catalase, and glutathione, and reduce seizure frequency in compare to placebo group. However, the mean of changes in the level of malondialdehyde, which is a product of lipid peroxidation, between Vitamin E and placebo groups was not significant. The differences between our findings and some previous studies can explain by different in antiepileptic therapy, whereas we enrolled patients who received any of AEDs and we did not compare variables between groups in regard to AEDs, but other studies analyzed their result in regard to AEDs. Also, that the dosage and treatment duration Vitamin E varies across studies. Vitamin E has been used in a wide dose ranges from modest 100 IU/d[32] or 200 IU/d[33,34] to high doses such as 1200 IU/d[35] in various experiments. As antiepileptics are commonly weak oxidants, and their influence on oxidative stress have been commonly traced after long term intake of this agents, we believe that administration of a modest (400 IU/d) dose of Vitamin E could sufficiently compensate for their pro-oxidant activity.
Although there is conflicting between researches on efficacy of Vitamin E and its exact pharmacological mechanism in response to diverse seizures remains elusive, in epileptic patients, Vitamin E is relatively safe and may be considered for adjunctive treatment.
The main limitation of our study was that we did not limited inclusion criteria to restricted AEDs so there were few patients in each individual AEDs groups and we could not analyzed efficacy of Vitamin E in regard to individual AEDs.
According tto Martinc et al. review article, In order to evaluate potentially positive effects of therapeutic interventions with antioxidant components, numerous studies of their use in animal models of epileptic seizures, were revised. However, even though numerous positive effects of antioxidants were observed in animal models of epileptic seizure, till now only few antioxidants have been further evaluated in patients with epilepsy as an add-on therapy and even these with only partial success. Based on the several positive findings in animal models, a strong need for more carefully planned, randomized, double-blind, cross-over, placebo-controlled clinical trials for the evaluation of antioxidants efficacy in patients with epilepsy is warranted.[36]
CONCLUSION
In conclusion, our results suggest that add-on Vitamin E in epileptic patients on anti-epileptic therapy by decreased in positive change in EEG findings, reduction in the seizure frequency and with its antioxidant and free radical scavenging property offering the advantage of reducing the oxidant stress and the following damage, can use as an adjunct to antiepileptic therapy but more research needs to be undertaken to demonstrate the exact effect of Vitamin E in added to antiepileptic therapy on the seizure frequency, and oxidative stress in epileptic patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Aguiar CC, Almeida AB, Araújo PV, de Abreu RN, Chaves EM, do Vale OC, et al. Oxidative stress and epilepsy: Literature review. Oxid Med Cell Longev 2012. 2012 doi: 10.1155/2012/795259. 795259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemade ST, Melinkeri R. Effect of antiepileptic drugs on antioxidant status epilepsy. Curr Neurobiol. 2010;1:109–12. [Google Scholar]
- 3.Schulpis KH, Lazaropoulou C, Regoutas S, Karikas GA, Margeli A, Tsakiris S, et al. Valproic acid monotherapy induces DNA oxidative damage. Toxicology. 2006;217:228–32. doi: 10.1016/j.tox.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Yis U, Seçkin E, Kurul SH, Kuralay F, Dirik E. Effects of epilepsy and valproic acid on oxidant status in children with idiopathic epilepsy. Epilepsy Res. 2009;84:232–7. doi: 10.1016/j.eplepsyres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Aycicek A, Iscan A. The effects of carbamazepine, valproic acid and phenobarbital on the oxidative and antioxidative balance in epileptic children. Eur Neurol. 2007;57:65–9. doi: 10.1159/000098053. [DOI] [PubMed] [Google Scholar]
- 6.Boon NA. Davidson's Principles and Practice of Medicine. 20th ed. USA: Churchill Livingstone, Elsevier; 2006. [Google Scholar]
- 7.Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahlen L. The effect of sodium valproat on folat levels, transmethylation, homocysteine and oxidative stress: Is there a link with neurodegeneration and continued seizure? Nutr Pract. 2011;12:36–60. [Google Scholar]
- 9.Vega Rasgado L, Ceballos Reyes G, Vega-Diaz M. Proceeding Western Pharmacology Society; 2011. Anticonvulsant Drugs, Oxidative Stress and Nitric Oxide. [PubMed] [Google Scholar]
- 10.Thanoon IA, Pachachi OA, Al-Sheikh MM. Effect of carbamazpin on serum leptin, insulin levels and oxidative stress in epileptic patients. Ann Coll Med Mosul. 2012;38:40–5. [Google Scholar]
- 11.Ogunmekan AO, Hwang PA. A randomized, double-blind, placebo-controlled, clinical trial of D-alpha-tocopheryl acetate (Vitamin E), as add-on therapy, for epilepsy in children. Epilepsia. 1989;30:84–9. doi: 10.1111/j.1528-1157.1989.tb05287.x. [DOI] [PubMed] [Google Scholar]
- 12.Nazıroğlu M. Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res. 2009;34:2181–91. doi: 10.1007/s11064-009-0015-8. [DOI] [PubMed] [Google Scholar]
- 13.Verrotti A, Scardapane A, Franzoni E, Manco R, Chiarelli F. Increased oxidative stress in epileptic children treated with valproic acid. Epilepsy Res. 2008;78:171–7. doi: 10.1016/j.eplepsyres.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Kovalenko VM, Kryzhanovskii GN, Kovalenko VS, Pronina IG, Nikushkin EV. Alpha-tocopherol in the complex treatment of several forms of epilepsy. Zh Nevropatol Psikhiatr Im S S Korsakova. 1984;84:892–7. [PubMed] [Google Scholar]
- 15.Ebrahimi H, Shafa M, Hakimzadeh Asl S. Prevalence of active epilepsy in Kerman, Iran: A house based survey. Acta Neurol Taiwan. 2012;21:115–24. [PubMed] [Google Scholar]
- 16.Mohammadi MR, Ghanizadeh A, Davidian H, Mohammadi M, Norouzian M. Prevalence of epilepsy and comorbidity of psychiatric disorders in Iran. Seizure. 2006;15:476–82. doi: 10.1016/j.seizure.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 18.Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196:143–51. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 19.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 20.Asif M. Role of various Vitamins in the patients with epilepsy. IJPR. 2013;3:34. [Google Scholar]
- 21.Higashi A, Tamari H, Ikeda T, Ohtani Y, Matsukura M, Miyoshino S, et al. Serum Vitamin E concentration in patients with severe multiple handicaps treated with anticonvulsants. Pediatr Pharmacol (New York) 1980;1:129–34. [PubMed] [Google Scholar]
- 22.Sudha K, Rao AV, Rao A. Oxidative stress and antioxidants in epilepsy. Clin Chim Acta. 2001;303:19–24. doi: 10.1016/s0009-8981(00)00337-5. [DOI] [PubMed] [Google Scholar]
- 23.Veinbergs I, Mallory M, Sagara Y, Masliah E. Vitamin E supplementation prevents spatial learning deficits and dendritic alterations in aged apolipoprotein E-deficient mice. Eur J Neurosci. 2000;12:4541–6. [PubMed] [Google Scholar]
- 24.deficits and dendritic alterations in aged apolipoprotein E-deficient mice. Eur. J. Neurosci. 2000;12:45414–16. [PubMed] [Google Scholar]
- 25.Levy SL, Burnham WM, Hwang PA. An evaluation of the anticonvulsant effects of Vitamin E. Epilepsy Res. 1990;6:12–7. doi: 10.1016/0920-1211(90)90003-e. [DOI] [PubMed] [Google Scholar]
- 26.Raju GB, Behari M, Prasad K, Ahuja GK. Randomized, double-blind, placebo-controlled, clinical trial of D-alpha-tocopherol (Vitamin E) as add-on therapy in uncontrolled epilepsy. Epilepsia. 1994;35:368–72. doi: 10.1111/j.1528-1157.1994.tb02446.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaya M, Cimen V, Kalayci R, Kucuk M, Gurses C, Arican N, et al. Catalase and alpha-tocopherol attenuate blood-brain barrier breakdown in pentylenetetrazole-induced epileptic seizures in acute hyperglycaemic rats. Pharmacol Res. 2002;45:129–33. doi: 10.1006/phrs.2001.0915. [DOI] [PubMed] [Google Scholar]
- 28.Tomé AR, Feng D, Freitas RM. The effects of alpha-tocopherol on hippocampal oxidative stress prior to in pilocarpine-induced seizures. Neurochem Res. 2010;35:580–7. doi: 10.1007/s11064-009-0102-x. [DOI] [PubMed] [Google Scholar]
- 29.Harma M, Harma M, Erel O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med Wkly. 2003;133:563–6. doi: 10.4414/smw.2003.10397. [DOI] [PubMed] [Google Scholar]
- 30.Levy SL, Burnham WM, Bishai A, Hwang PA. The anticonvulsant effects of Vitamin E: A further evaluation. Can J Neurol Sci. 1992;19:201–3. [PubMed] [Google Scholar]
- 31.Kim HC, Choi DY, Jhoo WK, Lee DW, Koo CH, Kim C. Aspalatone, a new antiplatelet agent, attenuates the neurotoxicity induced by kainic acid in the rat. Life Sci. 1997;61:PL 373–81. doi: 10.1016/s0024-3205(97)00963-6. [DOI] [PubMed] [Google Scholar]
- 32.Jain SK, McVie R, Jaramillo JJ, Palmer M, Smith T, Meachum ZD, et al. The effect of modest Vitamin E supplementation on lipid peroxidation products and other cardiovascular risk factors in diabetic patients. Lipids. 1996;31(Suppl):S87–90. doi: 10.1007/BF02637057. [DOI] [PubMed] [Google Scholar]
- 33.Tayebi Khosroshahi H, Habibi Asl B, Habibzadeh A, Chaichi P, Ghanbarpour A, Hossein Badie A. Comparison of Vitamin E and L-carnitine, separately or in combination in patients with intradialytic complications. Nephrourol Mon. 2013;5:862–5. doi: 10.5812/numonthly.10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Araoud M, Neffeti F, Douki W, Khaled L, Najjar MF, Kenani A, et al. Toxic effects of methamidophos on paraoxonase 1 activity and on rat kidney and liver and ameliorating effects of alpha-tocopherol. Environ Toxicol. 2014 doi: 10.1002/tox.22095. [DOI] [PubMed] [Google Scholar]
- 35.Singh U, Otvos J, Dasgupta A, de Lemos JA, Devaraj S, Jialal I. High-dose alpha-tocopherol therapy does not affect HDL subfractions in patients with coronary artery disease on statin therapy. Clin Chem. 2007;53:525–8. doi: 10.1373/clinchem.2006.078865. [DOI] [PubMed] [Google Scholar]
- 36.Martinc B, Grabnar I, Vovk T. Antioxidants as a preventive treatment for epileptic process: A review of the current status. Curr Neuropharmacol. 2014;12:527–50. doi: 10.2174/1570159X12666140923205715. [DOI] [PMC free article] [PubMed] [Google Scholar]


