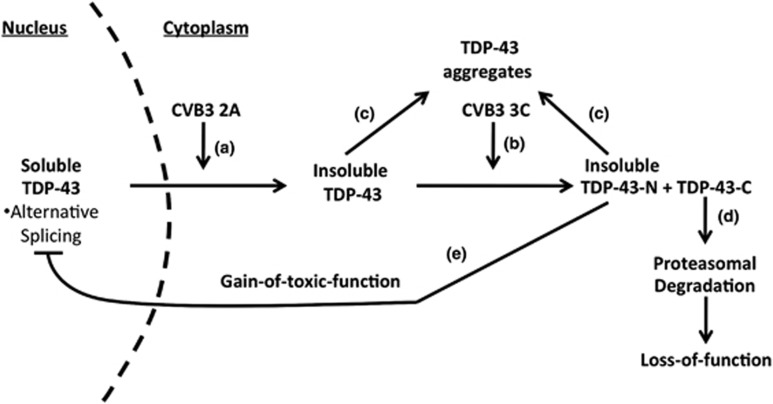
Figure 9.
Proposed model of CVB3 manipulation of TDP-43 pathway. Nuclear TDP-43 participates in the regulation of RNA metabolism under normal condition. Upon CVB3 infection, viral protease 2A causes the translocation of TDP-43 from the nucleus to the cytoplasm (a), where TDP-43 is cleaved at amino-acid Q327 by viral protease 3C to generate TDP-43-N and TDP-43-C (b). The solubility of cytoplasmic full-length TDP-43 and TDP-43-N is reduced and prone to aggregation (c). TDP-43-C is rapidly degraded via the proteasomal degradation pathway, leading to its loss-of-function (d), whereas TDP-43-N functions as a dominant-negative mutant, which inhibits the biological activity of TDP-43 in RNA splicing (e)