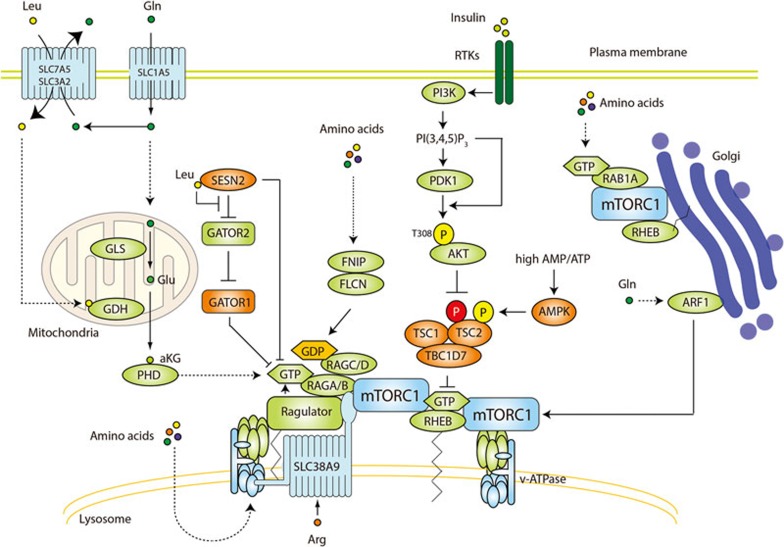
Figure 1.
Regulation of mTORC1. mTORC1 is activated by growth factors (such as insulin), cellular energy (ATP), and nutrients (amino acids). Growth factors activate the PI3K-PDK1-AKT pathway to inhibit the TSC complex, a GAP for RHEB. Upon inhibition of the TSC complex, GTP-bound RHEB binds and activates mTORC1 on the lysosome. Low cellular energy (high AMP/ATP ratio) stimulates AMPK to phosphorylate and activate the TSC complex, thus inhibiting RHEB and mTORC1. Amino acids promote activation of the RAG proteins to recruit mTORC1 to the lysosomal surface where it encounters RHEB. The GTPase RAGA or RAGB (RAGA/B) forms a heterodimer with the GTPase RAGC or RAGD (RAGC/D). Amino acids activate the RAG heterodimer by modulating its guanine nucleotide binding status. The active RAG heterodimer contains GTP-bound RAGA/B and GDP-bound RAGC/D. The heteropentameric RAGULATOR complex anchors the RAGs to the lysosomal surface, and is a GEF for RAGA/B. Upstream of RAGULATOR/RAGs, glutaminolysis promotes GTP loading of RAGB. The guanine nucleotide status of RAGA/B is also regulated by the GATOR1 complex (GAP) and its negative regulator GATOR2. SESN2 is a negative regulator of both GATOR2 and RAGA/B, in the latter case as a GDI. SESN2 is reported to be a cytoplasmic leucine sensor. Leucine binds to SESN2 and inhibits SESN2-GATOR2 interaction. The FLCN-FNIP complex is a GAP for RAGC/D. SLC38A9 is an arginine transporter in the lysosomal membrane and interacts with the RAGs and RAGULATOR to activate mTORC1. Glutamine also promotes lysosomal translocation and activation of mTORC1 through ARF1 in a RAG-independent manner. V-ATPase is required for lysosomal recruitment and activation of mTORC1 in RAG-dependent and -independent manners. Amino acids also activate mTORC1 via recruitment to the Golgi, involving RAB1A and Golgi-resident RHEB. Phosphorylation represented in yellow and red indicates an activation and inhibitory signal, respectively. Arrows and bars represent activation and inhibition, respectively, of the downstream protein.