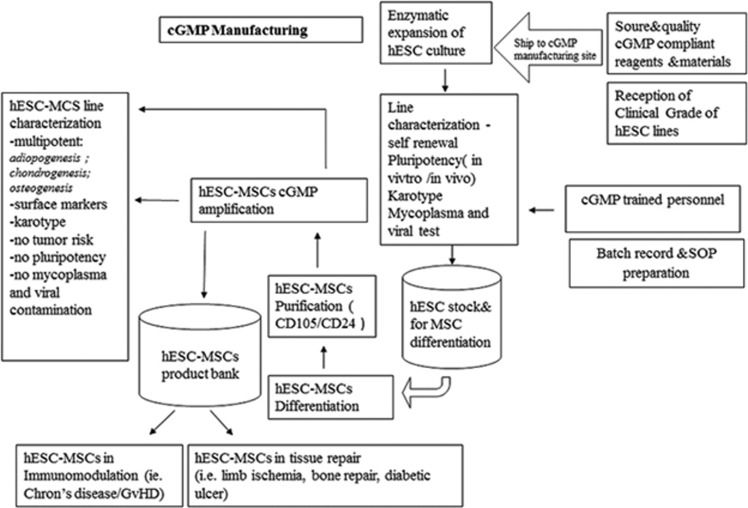
Figure 2.
Establishing clinical grade hESC-MSC lines under cGMP facilities and protocols. All parts of the process must be defined and operated by professionals: the cell lines, the starting materials, cell culture density and medium. Cells must be cultured under the GMP standard. Phenotype, functional potential and microbiological safety of each batch of hESC-MSCs are tested. Scientific, rigorous and complete quality control of cells should be done before infusion