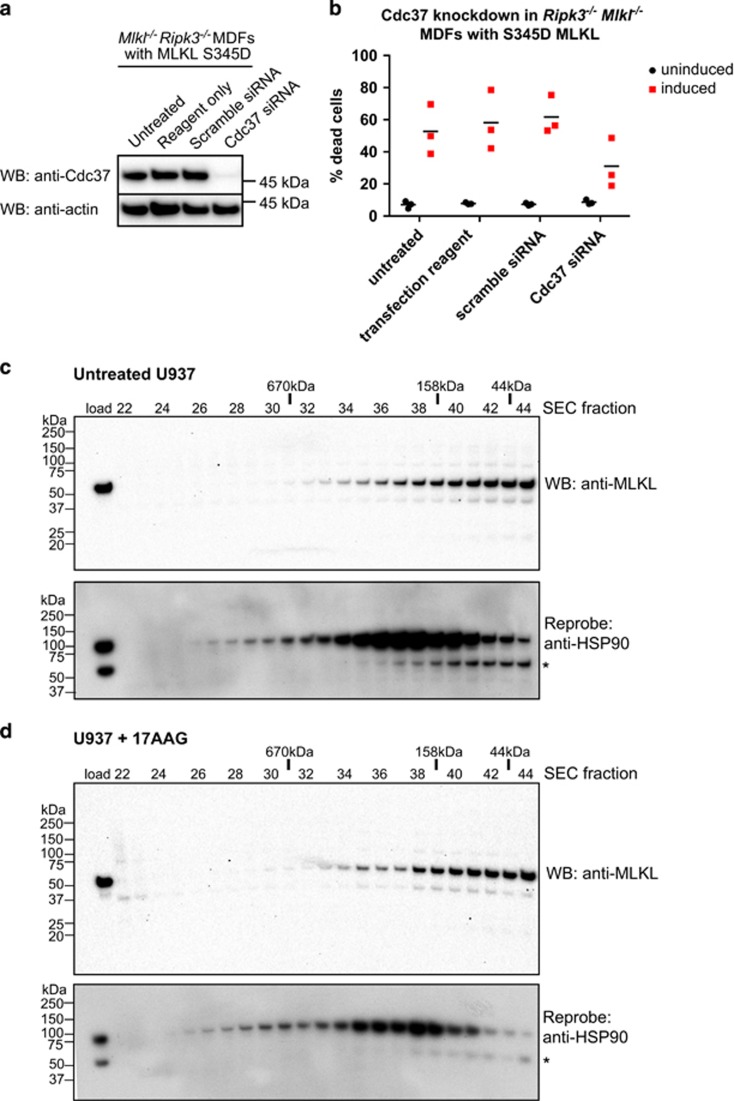
Figure 5.
MLKL transiently interacts with HSP90 via the Cdc37 co-chaperone. (a and b) Mlkl−/− Ripk3−/− MDFs stably transduced with a lentiviral construct encoding S345D MLKL were untreated, treated with transfection reagent only, or transfected with scrambled or Cdc37 siRNA pools. Cdc37 knockdown was observed in Cdc37 siRNA-treated cells relative to untreated, transfection reagent and scrambled siRNA-treated controls by western blot (a). Only Cdc37 siRNA knockdown conferred protection from S345D MLKL-mediated death (b). (c and d) Lysates of U937 cells incubated with DMSO (c) or 17-AAG (500 nM, d) were resolved by Superose-6 10/300 size-exclusion chromatography (SEC). Fractions were subjected to SDS-PAGE and western blotted for MLKL (upper panels) before reprobing for HSP90 (lower; * corresponds to residual signal from MLKL blots). The SEC fraction number is shown above the blots along with the elution position of molecular weight standards