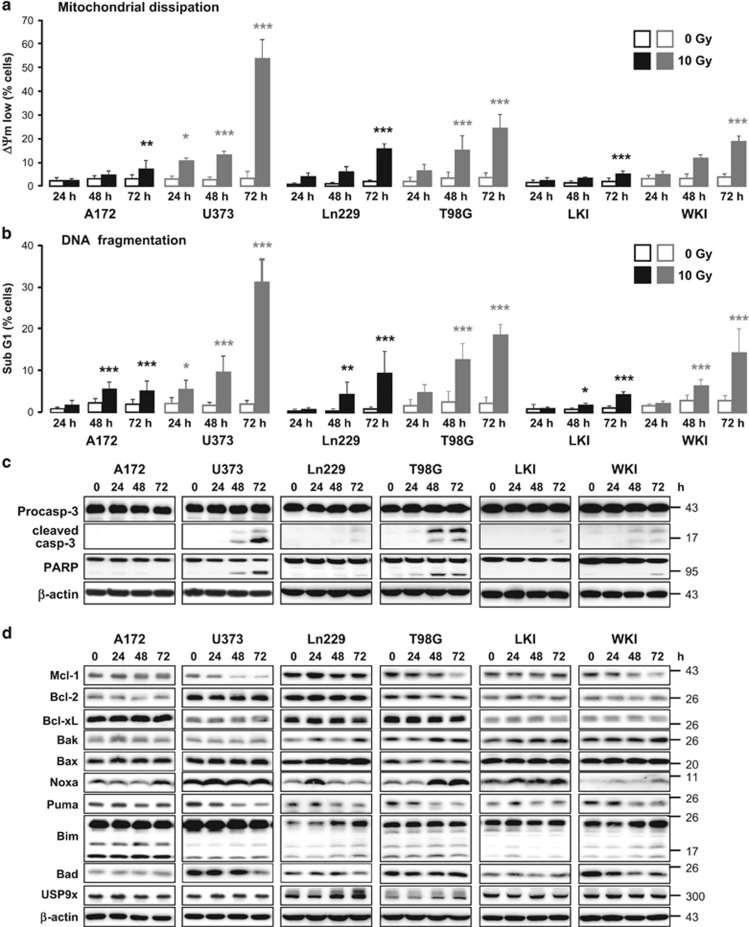
Figure 3.
Ionizing radiation induced downregulation of Mcl-1 and caspase-dependent apoptosis in U373, T98G, and WKI cells. A172, U373, Ln229, T98G, LKI, and WKI glioblastoma cells were irradiated with 0 Gy or 10 Gy. 24 h, 48 h, and 72 h after irradiation, dissipation of mitochondrial membrane potential (ΔΨm low, a) and DNA fragmentation (sub G1, b) were analyzed by flow cytometry. 72 h after irradiation, dissipation of ΔΨm and DNA fragmentation were clearly increased in U373, T98G, and, to a lesser extent, in WKI cells. Flow cytometric data show mean values±S.D. (n≥6). Significance was calculated to the respective non-irradiated control cells. *P<0.05, **P<0.01, ***P<0.001. (c, d) 0–72 h after irradiating glioblastoma cells, lysates were made and analyzed by western blot. β-actin was used as loading control. (c) Caspase-3 and PARP cleavage were detected only in U373, T98G, and WKI cells 48 and 72 h after irradiation, indicating apoptosis induction in response to IR in these cells but not in A172, Ln229, and LKI cells. (d) Protein levels of antiapoptotic Mcl-1 were downregulated in U373, T98G, and WKI cells 48 and 72 h after irradiation. The Mcl-1 decay correlated with cpase-3 and PARP cleavage. No change of antiapoptotic Bcl-2 and Bcl-xL levels was observed upon irradiation. The regulation of the pro-apoptotic proteins in response to IR differed between the cell lines, but it did not correlate with apoptosis induction