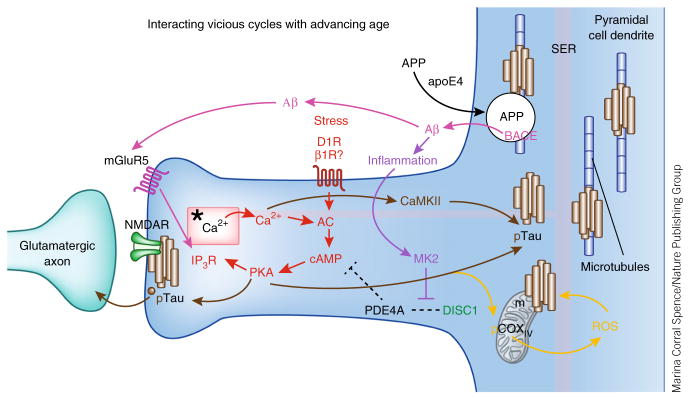
Figure 5.
The multiple, interacting, feedforward vicious cycles that may be disinhibited in the aging dlPFC, contributing to increased vulnerability to degeneration. Red: stress activates feedforward Ca2+–cAMP signaling pathways near the glutamate NMDAR synapses on spines. In the young adult dlPFC, the phosphodiesterase PDE4A is anchored by DISC1 next to the spine apparatus (*), an extension of the smooth endoplasmic reticulum (SER), critically positioned to regulate feedforward Ca2+–cAMP signaling in dlPFC spines. PDE4A is lost from spines with advancing age, dysregulating Ca2+–cAMP signaling and increasing the activation of kinases (for example, PKA and calcium/calmodulin-dependent kinase II (CaMKII)) that phosphorylate tau14. IP3R, inositol-1,4,5-trisphosphate receptor. Brown: pTau aggregates over the spine apparatus, at glutamatergic synapses, and over microtubules in dendrites and traffics in vesicles between neurons14. The aggregation of pTau on microtubules in dendrites likely interferes with intracellular trafficking, including the trafficking of APP, the precursor to Aβ. Magenta: APP is cleaved to Aβ when it is trapped in endosomes that contain β-secretase (BACE)—for example, when there is interference with APP endosomal trafficking143. Indeed, the increased risk of Alzheimer’s disease conferred by the apoE4 variant is thought to involve increased localization of APP into endosomes145. The aggregation of pTau on microtubules may similarly trap APP-containing endosomes and lead to the increased generation of Aβ oligomers. The generation of Aβ oligomers can drive additional vicious cycles by stimulating mGluR5 (ref. 147). mGluR5 are localized near the synapse on spines in dlPFC, positioned to activate feedforward Ca2+–cAMP signaling and thus drive more tau phosphorylation. Purple: Aβ fibrils drive inflammation148, which can unanchor residual PDE4A125 and further disinhibit stress signaling pathways. Orange: increased stress signaling may also dysregulate mitochondrial function, as PKA can phosphorylate cyclooxygenase IV (COXIV) to increase reactive oxygen species (ROS)149, which also increase tau phosphorylation and Aβ production150, leading to additional mitochondrial dysfunction. Thus, dysregulation of stress signaling pathways in the dlPFC with advancing age may contribute to many deleterious molecular events that increase vulnerability to degeneration. Alzheimer’s disease pathology may begin anywhere along these pathways (for example, genetic alterations in APP processing or environmental stressors promoting pTau) and, by driving these interacting cycles, lead to the same degenerative phenotype.