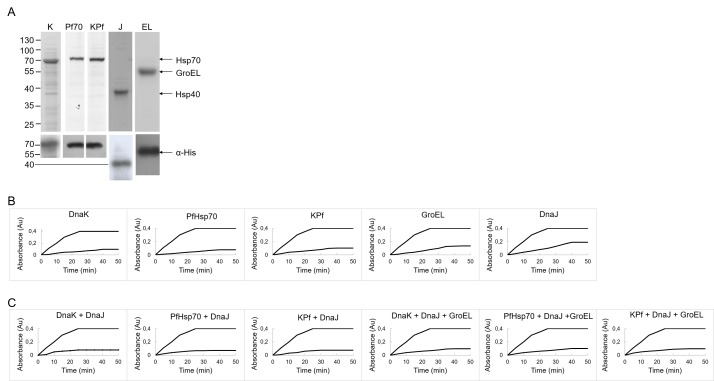
Fig 8. Suppression of heat-induced aggregation of MDH by molecular chaperones.
(A) SDS-PAGE analysis confirming the purification of His-tagged DnaK, PfHsp70, KPf, DnaJ and GroEL-GroES overproduced in E. coli XL1 Blue cells and Western analysis using α-His antibody to detect the proteins. Numbers to the left indicate protein markers (Fermentas) in kDa. Recombinant forms of DnaJ, DnaK, KPf, PfHsp70 and GroEL-GroES at a final concentration of 0.36 μM, either ndependently (B) or in combination (C) were assessed for their ability to inhibit heat-induced aggregation of MDH. The heat induced aggregation of 0.72 μM MDH was monitored at 48°C and 360 nm for 50 mins. The experiment was conducted in the absence or presence of the respective chaperones as indicated.