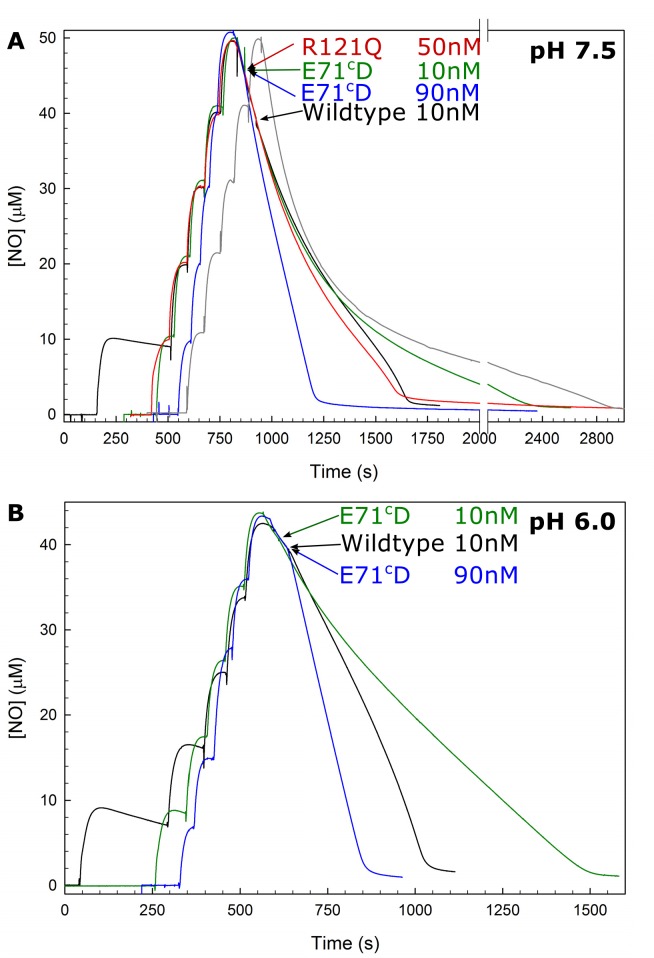
Fig 5. Multiple turnover activity of cNOR variant E71cD with NO is not sigmoidal.
The NO reduction by the E71cD variant is shown at two concentrations: 10 nM (green line) and 90 nM (blue line) at pH 7.5 (A), and at pH 6.0 (B). For comparison data for 10 nM wildtype (black line), 50 nM R121Q (red line, only in A), and background consumption (no enzyme added, grey line in A) is also shown. R121Q has a lower multiple turnover activity, but is still sigmoidal. Experimental conditions: T = 295K, NO-saturated water (2 mM NO) was added in five steps of 5 μl (10 μM/addition) to a deoxygenated solution of 50 mM HEPES, pH 7.5, 50 mM KCl, 0.05% (w/v) DDM, 30 mM glucose, 20 units/ml catalase, 1 unit/ml glucose oxidase, 500 μM TMPD, and 20 μM horse heart cytochrome c. Then 3 mM ascorbate was added (giving some background NO reduction), followed by cNOR addition (as indicated by arrow and label). Note that the background trace shown in A was recorded at 303K, whereas the traces with cNOR were recorded at 295K, which explains the high background consumption at high [NO].