Figure 6.
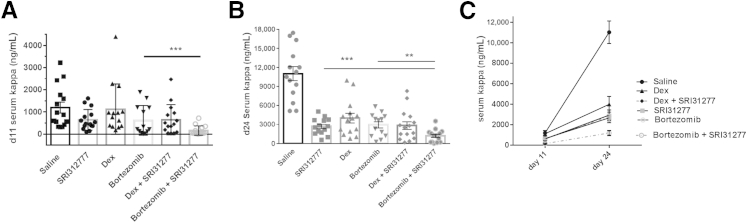
SRI31277 is as effective as either dexamethasone (dex) or bortezomib, and bortezomib and SRI31277 together have increased benefit in reducing myeloma tumor burden. Severe combined immunodeficiency mice were injected with heparanase-expressing CAG human myeloma cells expressing luciferase via tail vein. After 2 weeks, treatment with saline or 0.3 mg/kg per day SRI31277 delivered by osmotic pump was initiated. Some groups received via i.p. injection 1 mg/kg dexamethasone once per week or 1 mg/kg bortezomib twice per week. In addition, two groups were treated with SRI31277 + dexamethasone or SRI31277 + bortezomib (n = 15 per group). Treatments continued for approximately 4 weeks. A and B: Serum human Ig kappa levels were measured after 11 (A) and 24 (B) days of treatment. C: Tumor progression over time. Results are the means ± SEM with individual values plotted. For day 11, P = 0.00015, unpaired t-test with Welch correction. For day 24, P = 0.0034, bortezomib versus SR31277 + bortezomib; and P = 0.0017, SRI31277 versus SRI31277 + bortezomib, unpaired t-test. ∗∗P < 0.01, ∗∗∗P < 0.001.