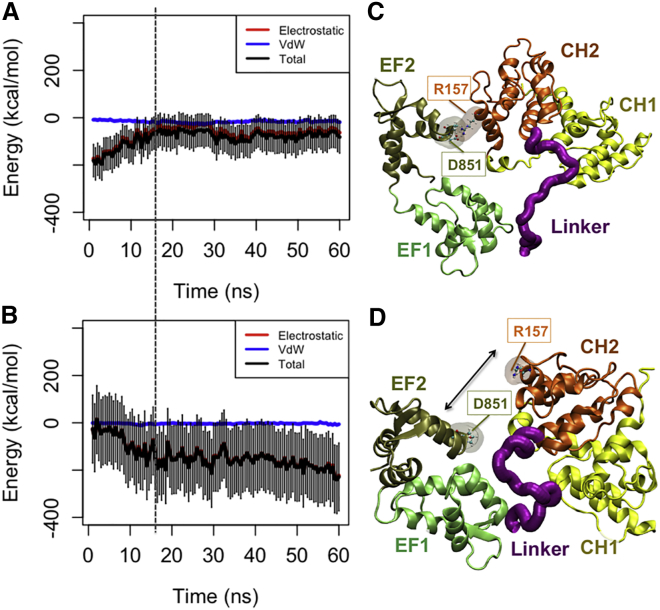
Figure 6.
The CaM domain was released from the neck region as CH2 associated with actin in the CW simulations. (A) The interaction energy between CaM and the neck region was reduced around 18 ns (indicated by the dashed line), as (B) the CH2 domain got engaged with actin. (C and D) In one of the three CW trials, a salt bridge between D851 in CaM and R157 in CH2 was released allowing CH2 to form a more effective interaction with actin. (C) A salt bridge between R157 and D851 linked the CaM domain and the cABD in two out of three CW trials (see Table 1). CH1 is loosely associated with the second actin monomer blocking it from binding to CH2. Furthermore, the CaM domain itself associated with actin only in the presence of the salt bridge. (D) The disruption of the salt bridge between R157 and D851 linked the CaM domain and the cABD in one of the CW simulations released from CaM and resulted in a more effective binding between CH2 and actin. Only, in this trial, CH2 engaged with both proximal actin monomers, and the interaction with the second monomer followed the disruption of the salt bridge. To see this figure in color, go online.