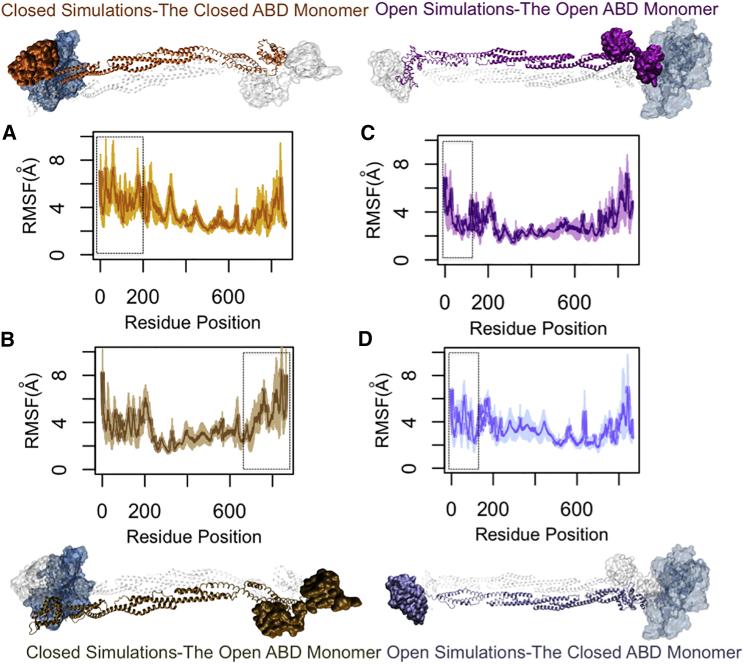
Figure 8.
The RMSFs of α-actinin monomers both in bound and unbound states to actin. The rod domain had the lowest level of fluctuations in both CW and OW simulations. (A) The cABD domain (residues 25–250) in the CW simulations (the orange α-actinin monomer) underwent a slightly lower fluctuation compared to (B), the open ABD that was relative to actin (the tan α-actinin monomer). However, the CaM domain near actin (the C-terminal of the monomer with open ABD) showed a similar level of fluctuations to the cABD of its neighboring monomer in the CW simulations (dashed boxes). (C) In the OW simulations, the RMSF of the CH1 domain of the oABD was lowered upon actin binding (the purple α-actinin monomer) and was clearly different from that of the CH2 domain in the same monomer, whereas (D) the closed ABD that was far from actin did not show any clear difference between RMSFs of CH1 and CH2 (the ice blue α-actinin monomer). All plots are averaged over three trials. To see this figure in color, go online.