Abstract
In this study, three typical members representative of different arginine metabolic pathways were firstly identified from Apostichopus japonicus, including nitric oxide synthase (NOS), arginase, and agmatinase. Spatial expression analysis revealed that the AjNOS transcript presented negative expression patterns relative to those of Ajarginase or Ajagmatinase in most detected tissues. Furthermore, Vibrio splendidus-challenged coelomocytes and intestine, and LPS-exposed primary coelomocytes could significantly induce AjNOS expression, followed by obviously inhibited Arginase and AjAgmatinase transcripts at the most detected time points. Silencing the three members with two specific siRNAs in vivo and in vitro collectively indicated that AjNOS not only compete with Ajarginase but also with Ajagmatinase in arginine metabolism. Interestingly, Ajarginase and Ajagmatinase displayed cooperative expression profiles in arginine utilization. More importantly, live pathogens of V. splendidus and Vibrio parahaemolyticus co-incubated with primary cells also induced NO production and suppressed arginase activity in a time-dependent at an appropriate multiplicity of infection (MOI) of 10, without non-pathogen Escherichia coli. When increasing the pathogen dose (MOI = 100), arginase activity was significantly elevated, and NO production was depressed, with a larger magnitude in V. splendidus co-incubation. The present study expands our understanding of the connection between arginine’s metabolic and immune responses in non-model invertebrates.
L-arginine is a crucial amino acid because it is involved in multiple metabolic pathways1,2, and plays important roles in physiological and pathological processes by producing a wide range of metabolites, including nitric oxide (NO), urea, creatinine, agmatine and polyamines3,4. It is therefore no surprise that its metabolic pathways are complex and highly regulated by different metabolites. The emerging importance of arginine is evident in many metabolic processes, such as the NO and polyamine biosynthesis pathways, in which arginine acts as a pivotal immune system regulator and helps modulate the immune response during infection5,6,7. Among them, nitric oxide synthase (NOS) and arginase are the most important enzymes that participate in inimitable catalytic steps with antagonistic roles linking arginine metabolism and the immune response8,9.
NOS and arginase, two classic immune regulator molecules, both have L-arginine as a common substrate and compete with each other for this substrate. Their involvement in arginine metabolism has been well described in mammalian immune systems7,10,11,12,13. There are three distinct isoforms of NOS, namely endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS)7,10. eNOS and nNOS are continuously expressed and regulated by Ca2+/Calmodulin14. The third type of iNOS is not continuously presented but is highly induced by pathogens or bacterial components, such as lipopolysaccharide (LPS) and immunostimulation, with Ca2+-independent regulation15,16. NO is the central component produced by three isoforms of the NOSs using L-arginine as the exclusive physiological substrate and L-citrulline as a co-product10,11. It is both a gasotransmitter and an important signaling molecule, which is predominantly associated with antimicrobials in the immune system and is biosynthesized in many immunocytes, including macrophages, neutrophils, monocytes, and endothelial cells17,18,19,20. The depletion of arginine as a means of increasing NO production is a beneficial strategy employed by host cells in order to kill invasive bacteria, viruses and parasites17,21,22. In recent years, the three isoforms of NOSs have been obtained and described from many vertebrate species23,24, whereas only one NOS gene has been reported in most invertebrate genomes21,25. In marine invertebrates, the NOS gene has been identified from shrimp Litopenaeus vannamei and scallop Chlamys farreri after LPS or Vibrio harveyi exposure, and their roles in immune defense are well indicated26,27,28. Unfortunately, no evidence shows the other pathways of arginine metabolism, such as the arginase pathway, and whether it competes with the NOS/NO pathway is still largely a mystery in invertebrates.
In vertebrates, if the arginine metabolic pathway is controlled by arginase, the results would be completely opposite. Arginase, which has two isoforms (arginase I and arginase II), is one of the enzymes that competes with NOSs for L-arginine, which is a substrate produces ornithine and urea and reciprocally modulates NOS activity29. The hydrolysis of arginine through the arginase pathway will result in polyamine biosynthesis and lead to decreased bactericidal NO production. Additionally, it will increase the growth of bacterial and parasitic pathogens because polyamines play an important role in cell growth and proliferation, which is harmful to host tissues and cells7,30,31. Bussiere et al. demonstrated that the metabolite of spermine from the arginase pathway could prevent the antimicrobial effects of NO by inhibiting iNOS translation in macrophages infected by Helicobacter pylori32. Amazingly, H. pylori could use its own arginase (RocF) to evade the antimicrobial effects of macrophage-derived NO through competition using the common substrate L-arginine from the host33. Numerous studies have indicated that arginase and NOS play antagonistic roles during the immune response8,31. Inhibition or knockout of arginase is known to significantly increase NO production, which depends on NOS3,34. Interestingly, the metabolism of L-arginine to polyamines via agmatinase is an alternative pathway long recognized in lower organisms, which first degrades arginine to agmatine via arginine decarboxylase (ADC)35,36. Agmatinase is a binuclear manganese metalloenzyme and belongs to the ureohydrolase superfamily, which includes arginase, formiminoglutamase and proclavaminate amidino hydrolase37. It is well known that agmatinase shares regions of strong sequence homology with authentic arginase and acts as an intermediate in arginine metabolism of various lower organisms and mammals38,39. Agmatine not only competitively inhibits three isoenzymes of NOSs but also significantly inhibits polyamine synthesis catalyzed by agmatinase40,41,42. Currently, the metabolic responses of arginine and the modulation of its production through the NOS/NO or arginase/polyamines pathways, as well as the alternative pathway, have been well documented in vertebrate immune response, especially in mice and humans. However, whether these differentially expressed molecules exist in invertebrates remains largely unknown, and their roles acting in arginine metabolism under pathogen infection or disease outbreaks are poorly understood. Thus, the exploration of complete arginine metabolic pathways in invertebrates is significant.
The invertebrate sea cucumber Apostichopus japonicus (Echinodermata, Holothuroidea), which has an innate immune system, is one of the most important economic marine species in Chinese aquaculture. In echinoderms, cell-based immunity is based on coelomocytes, a morphologically heterogeneous population with the capacity to recognize and neutralize pathogens. Unfortunately, the natural resources of A. japonicus in China have declined drastically due to various viral and bacterial disease outbreaks43,44 in which Vibrio was widely accepted as one of the major pathogens by many researchers, especially Vibrio splendidus and Vibrio parahaemolyticus. They are both Gram-negative halophilic and mesophilic bacteria and are commonly found in marine environments, causing diseases in marine animals45. In our previous work, we found that the metabolites of arginine uniquely increased in V. splendidus-challenged A. japonicus samples after infection for 96 h, whereas lower levels were detected in SUS-diseased sea cucumbers46. It is important to not only investigate the mechanisms of initiating an immune response but also gain a deeper understanding of the reasons why these reactions appear. Therefore, in our current study, we will first describe the three arginine metabolic pathways in sea cucumbers and understand their functional cooperation in allocating arginine during pathogen infection.
Results
Cloning and sequences analysis of the three genes
Three full-length cDNAs from the different arginine pathways were generated by overlapping the fragments from ESTs and using the RACE approach in the sea cucumber Apostichopus japonicus (denoted AjNOS, Ajarginase, and Ajagmatinase), which were deposited in GenBank with accession Nos. KT366016, KT724965 and KT366017, respectively. The cDNA sequence of AjNOS was 5957 bp in length and contained an ORF of 5313 bp encoding a predicted product with 1770 amino acid residues with a molecular weight of 197.02 kDa and a theoretical pI of 6.23 (Fig. S1). The 5′-UTR was 68 bp long, and the 3′-UTR of 576 bp contained a polyadenylation signal (AATAAA) and RNA instability sequences (ATTTA). Multiple alignments showed that the deduced amino acid sequence of AjNOS shared greater identity with the NOS family, such as 52% homology with Strongylocentrotus purpuratus NOS (XP_011665134.1), 52% homology with Homo sapiens eNOS (AAH69465.1), 46% homology with H. sapiens iNOS (BAA37123.1), and 45% homology with H. sapiens nNOS (AAB60654.1). The representative domains of NOS from low invertebrate to human were totally conserved in the deduced amino acid of AjNOS, including the PDZ domain, N-terminal oxygenase domain, C-terminal reductase domain and a CaM binding site (Fig. S2). The Ajarginase cDNA transcript was 1860 bp and consisted of an ORF encoding a protein sequence of 384 amino acid residues with a predicted molecular weight of 41.71 kDa, and a theoretical pI of 6.54 (Fig. S3). The AjAagmatinase cDNA transcript comprised 1411 bp, including a 246 bp 5′-UTR, a 103 bp 3′-UTR with one RNA instability sequence (ATTTA) encoding 353 amino acid residues (Fig. S4). The predicted molecular mass of the deduced amino acids of Ajagmatinase was 38.64 kDa, and its theoretical pI was 6.46. Amino acid sequences alignment between Ajarginase and Ajagmatinase both from the ureohydrolase superfamily, showed only 18% identity with the highly conserved region of an arginase domain (Fig. S5). Among the conserved region are the two manganese-ion-binding sites present in these proteins. Sea cucumber agmatinase had the highest amino acid identity and similarity with C. gigas agmatinase (XP_011443425.1), although the overall sequence homology with human arginase 1 and arginase 2 was only 21% and 18%, respectively. To determine the evolutionary position of AjNOS, Ajarginase and Ajagmatinase from different arginine metabolic pathways, phylogenetic trees were constructed using the NJ method. The results showed that all three typical members shared greater homology to their counterparts from vertebrates. AjNOS was first clustered with other invertebrates from S. purpuratus, C. gigas (XP_011420158.1) and Aplysia california (NP_001191470.1), and then clustered with vertebrate nNOS to form a separated clade, which indicated that AjNOS belong to the nNOS subfamily (Fig. S6). Ajagmatinase was first grouped with invertebrate and vertebrate agmatinase and formed an independent clade; it was then grouped with bacteria agmatinase, which were clearly distinguished from the arginase subfamily, with the Vibrio parahaemolyticus arginase used an out group (Fig. S7). This phylogenetic tree analysis suggested that arginases and agmatinases from different species were derived from a common ancestor.
Tissue distribution of AjNOS, Ajarginase and Ajagmatinase
The constitutive expression of AjNOS, Ajarginase and Ajagmatinase in different tissues was investigated by quantitative real-time PCR, and the expression levels in muscle served as a reference. The results showed that the three genes were ubiquitously expressed in all examined tissues (Fig. 1). The mRNA transcript of AjNOS was expressed most strongly in the intestine (14.95-fold), followed by the tentacle (11.82-fold), respiratory tree (9.58-fold) and coelomocytes (4.43-fold). Ajarginase and Ajagmatinase displayed almost opposite expression profiles as AjNOS. Ajarginase and Ajagmatinase both presented relative lower expression levels in four other tissues including coelomocytes (0.21-fold and 0.12-fold), intestine (0.17-fold and 0.11-fold), tentacle (009-fold and 0.20-fold), and respiratory tree (0.43-fold and 0.74-fold) when compared with muscle.
Figure 1. The tissue distribution of AjNOS, Ajarginase and Ajagmatinase in normal sea cucumber detected by quantitative PCR.
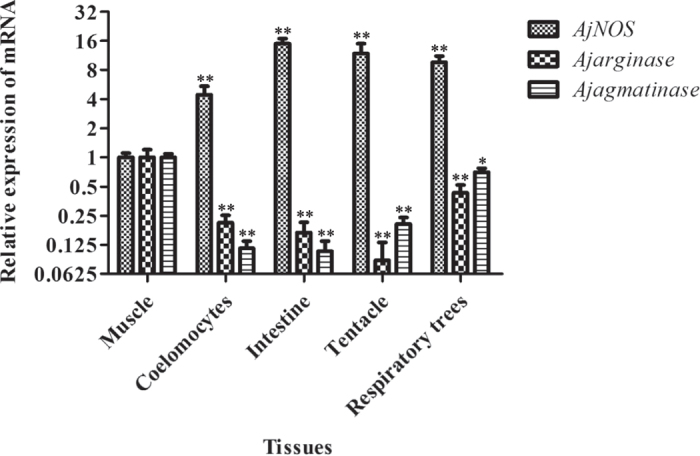
The transcript levels in coelomocytes, intestine, tentacle, and respiratory tress were normalized to that in muscle. Five biological replicates were performed in the experiment and the obtained data were expressed as the mean ± SD (n = 5). Asterisks indicated significant differences: *P < 0.05, **P < 0.01.
Time-course expression of AjNOS, Ajarginase and Ajagmatinase in vivo and in vitro
After the stimulation of V. splendidus, the temporal mRNA expression of AjNOS, Ajarginase and Ajagmatinase in coelomocytes and intestine is shown in Fig. 2. The expression level of AjNOS mRNA in coelomocytes (Fig. 2A) was gradually increased at 24 h post-infection, which sharply increased and reached peak expression at 96 h with a 7.53-fold (P < 0.01) increase compared to that in the control group throughout the experiment. The expression profiles of Ajarginase and Ajagmatinase were completely different from that of AjNOS. The mRNA expression levels of Ajarginase and Ajagmatinase remained at the control level at the first 6 h. Subsequently, the mRNA levels of Ajarginase and Ajagmatinase were both fleetly decreased and reached their lowest mRNA levels at 48 h with 0.40-fold (P < 0.05) and 0.50-fold (P < 0.05) decreases, respectively, which were still lower than the original level, with a lower magnitude in Ajarginase until the end of the test compared with the control group. When in the intestine (Fig. 2B), the level of the AjNOS transcript was also significantly increased at each detected time point compared with controls and occurred earlier than that of coelomocytes after V. splendidus infection, although the increased magnitude was lower than in coelomocytes. Consistently, the expression level of Ajarginase was tightly correlated with that of the coelomocyte Ajarginase, which also presented an opposite trends of expression to that of AjNOS in intestinal tissue. In contrast, Ajagmatinase was expressed at a higher level at 6 h, with a 1.67-fold (P < 0.05) compared with the control. Moreover, at 48 h, its expression was decreased 0.32-fold (P < 0.05), but it was still lower than the original level at 96 h compared with the control group.
Figure 2. Time-course expression of AjNOS, Ajarginase and Ajagmatinase in coelomocytes and intestine after Vibrio splendidus infection.
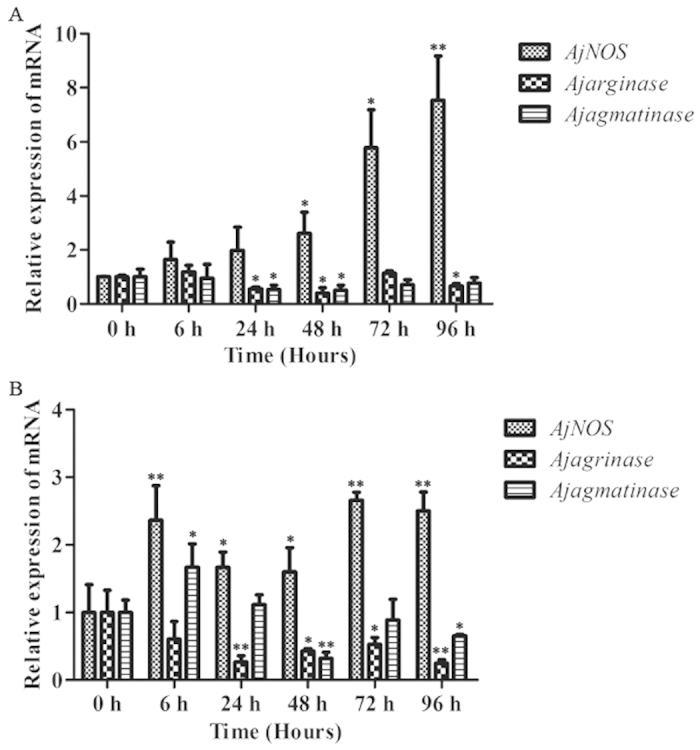
Five biological replicates were performed in the experiment and the obtained data were expressed as the mean ± SD (n = 5). Asterisks indicated significant differences: *P < 0.05, **P < 0.01.
The expression profiles of the three genes in primary coelomocytes after exposure to LPS in vitro were summarized in Fig. 3. At an LPS concentration of 10 μg mL−1, transcription of AjNOS remained at the control level for the first 3 h, then increased significantly (1.76-fold, P < 0.05) at 6 h and reached the highest expression at 24 h (2.08-fold, P < 0.01) compared with the control group. The level of the Ajarginase transcript was down regulated after 3 h (0.64-fold, P < 0.05) and reached its lowest expression at 12 h with a 0.42-fold decrease (P < 0.01). However, the expression profile of AjAgmatinase was not significantly changed at all examined time points during LPS challenge.
Figure 3. Transcriptional regulation of AjNOS, Ajarginase and Ajagmatinase in LPS-exposed coelomocytes at 0, 3, 6, 12 and 24 h.
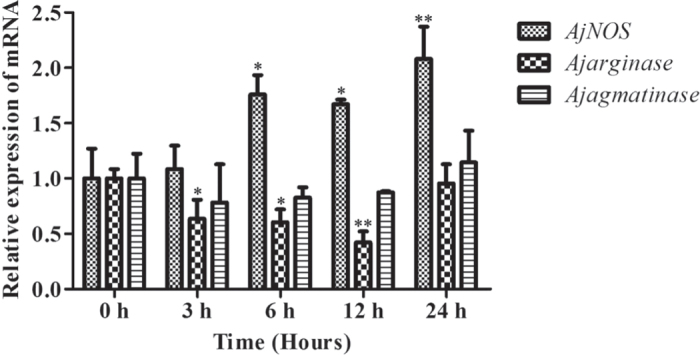
Five biological replicates were performed in the experiment and the obtained data were expressed as the mean ± SD (n = 5). Asterisks indicated significant differences: *P < 0.05, **P < 0.01.
NO production and arginase activities following AjNOS, Ajarginase or Ajagmatinase silencing in vitro
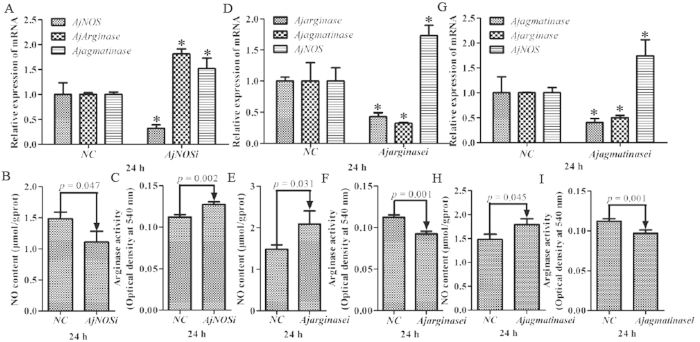
We transfected two specific siRNAs for each gene, and the efficiency of RNAi-mediated transcript depletion in primary cells was determined by quantitative RT-PCR. The interference efficiency of each gene, NO production and arginase activity were taken as the average after two siRNA transfections. Our results showed that AjNOS, Ajarginase and AjAgmatinase mRNA transcripts were inhibited by more than 52% after specific siRNA transfection (Fig. 4A,D,G). For AjNOS interference, the NO content of primary cells was markedly decreased by 25.1% (P = 0.047) compared with the negative control (Fig. 4B). In contrast, the mRNA expression of Ajarginase and Ajagmatinase was significantly increased by 1.81 fold (P < 0.05) and 1.52-fold (P < 0.05) (Fig. 4A), respectively, and the arginase activity also showed a positive correlation with the mRNA expression, which increased by 13.4% (P = 0.002) after AjNOS knock-down (Fig. 4C). Moreover, the AjNOS transcript was dramatically up-regulated 1.73-fold (P < 0.05) and 1.74-fold (P < 0.05), and the NO production increased by 40.6% (P = 0.031) and 20.6% (P = 0.045) after Ajarginase and Ajagmatinase silencing for 24 h, respectively (Fig. 4E,H). Arginase activity was tightly correlated with the expression levels of Ajarginase and Ajagmatinase and decreased by 17.7% (P = 0.001) and 13.5% (P = 0.001), respectively, after interference (Fig. 4F,I).
Figure 4. Related data from Apostichopus japonicus primary cultured coelomocytes after each gene silencing.
(A,D,G) Silencing efficiency of AjNOS, Ajarginase or Ajagmatinase in primary coelomocytes after specific siRNAs transfection and relative expression of mRNAs after interfering for 24 h, respectively. (B,E,H) NO production in the primary cultured coelomocytes after AjNOS, Ajarginase or Ajagmatinase knock-down, respectively. (C,F,I) arginase activity in the primary cultured coelomocytes after AjNOS, Ajarginase or Ajagmatinase knock-down, respectively. Five biological replicates were performed in the experiment and the obtained data were expressed as the mean ± SD (n = 5).
NO production and arginase activities following AjNOS, Ajarginase or Ajagmatinase silencing in vivo
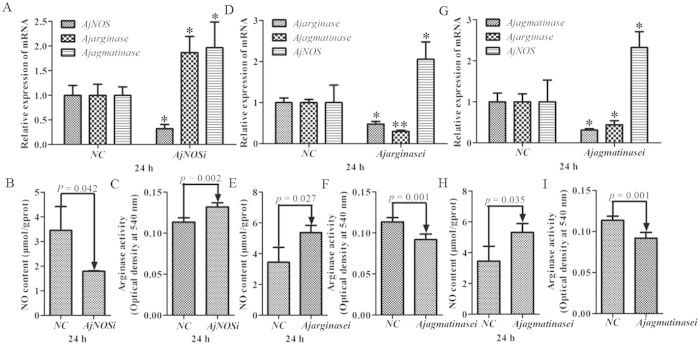
Figure 5 shows the mRNA expression levels of AjNOS, Ajarginase and Ajagmatinase, as well as NO production and arginase activity after the silencing of three genes in individual coelomocytes. The data were processed in vivo as well as in vitro. Three genes were significantly inhibited by more than 55% after specific siRNA transfection (Fig. 5A,D,G). The results in Fig. 5A showed that the levels of Ajarginase and Ajagmatinase were increased by 1.87-fold (P < 0.05) and 1.97-fold (P < 0.05), respectively, in the AjNOS silenced sea cucumbers, followed by the decrease in NO generation by 48.0% (P = 0.042) and an increase in arginase activity by 16.1% (P = 0.002) (Fig. 5B,C). When the sea cucumbers were treated with Ajarginase or Ajagmatinase siRNAs, the experimental results showed that the level of AjNOS was markedly increased by 2.06-fold (P < 0.05) and 2.32-fold (P < 0.05) after Ajarginase or Ajagmatinase siRNAs, respectively, were injected into individuals (Fig. 5D,G). Meanwhile, the amount of NO production was increased by 55.9% (P = 0.027) and 49.9% (P = 0.035) in silenced sea cucumbers (Fig. 5E,H), and arginase activity was observed to be lower both in the Ajarginase (decreased by 23.6%, P = 0.001) and Ajagmatinase (decreased by 19.1%, P = 0.001) siRNA groups after 24 h silencing compared with the negative control (Fig. 5F,I). Moreover, the Ajarginase and Ajagmatinase transcripts displayed cooperative expression profiles after one of interference both in vivo and in vitro, respectively.
Figure 5. Related data from Apostichopus japonicus individual levels after each gene silencing.
(A,D,G) Silencing efficiency of AjNOS, Ajarginase or Ajagmatinase in individuals coelomocytes after specific siRNAs transfection and relative expression of mRNAs after interfering for 24 h, respectively. (B,E,H) NO production in the individuals coelomocytes after AjNOS, Ajarginase or Ajagmatinase knock-down, respectively. (C,F,I) arginase activity in the individuals coelomocytes after AjNOS, Ajarginase or Ajagmatinase knock-down, respectively. Five biological replicates were performed in the experiment and the obtained data were expressed as the mean ± SD (n = 5).
In vitro induction of NO production and arginase activity following pathogenic or non-pathogenic challenge
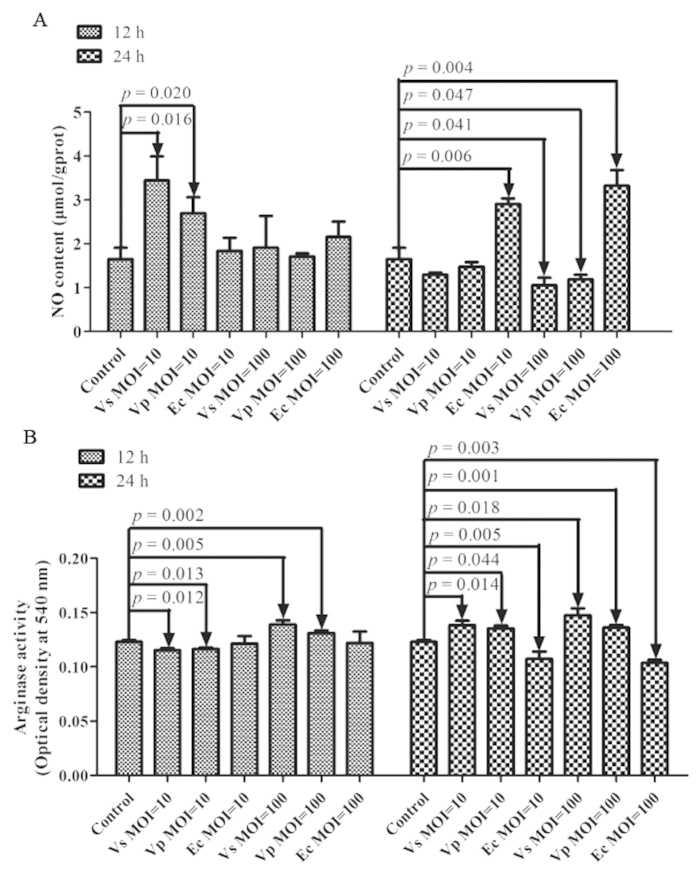
The NO production and arginase activity upon primary coelomocytes stimulation with pathogens or non-pathogens were generally dependent on the MOI employed and time point (Fig. 6). When primary cells were co-incubated with the pathogens V. splendidus and V. parahaemolyticus, NO production was markedly increased by 108.8% (P = 0.016) and 63.3% (P = 0.020), respectively, at 12 h and recovered to the original level at 24 h with an MOI of 10 (Fig. 6A). When challenge with two pathogens at an MOI of 100, NO production was gradually decreased by 36.2% (P = 0.041) and 27.8% (P = 0.047) for 24 h, respectively. There was no significant change in NO production and arginase activity after non-pathogen challenge of primary cells with E. coli with different MOIs at 12 h. However, NO production was sharply increased by 76.3% (P = 0.006) and 101.5% (P = 0.004) after 24 h with MOI = 10 and MOI = 100, followed by down-regulated arginase activity with 12.7% (P = 0.005)and 15.7% (P = 0.003), respectively. Furthermore, arginase activity was also found to be negatively correlated with NO production after pathogen challenge of primary cells in vitro (Fig. 6B). The arginase activity of sea cucumber coelomocytes exhibited the highest degree of sensitivity to V. splendidus. At an MOI of 10, the arginase activity first decreased by 6.2% (P = 0.012) in the V. splendidus group and 5.4% (P = 0.013) in the V. parahaemolyticus group at 12 h, and both induced significant response rates in 12.8% (P = 0.005) and 10.1% (P = 0.002), respectively, at 24 h compared to controls. When an MOI of 100, arginase activity was quickly up-regulated by 12.5% (P = 0.014) in the V. splendidus group and 6.7% (P = 0.044) in the V. parahaemolyticus group at 12 h and increased by 19.8% (P = 0.018) and 10.8% (P = 0.001) at 24 h after V. splendidus and V. parahaemolyticus, respectively, was co-incubated with primary cultured cells.
Figure 6. Effects of in vitro stimulation of pathogen or non-pathogen on Apostichopus japonicus primary cultured coelomocytes for 12 and 24 h with an appropriate multiplicity of infection (MOI) of 10 and 100.
(A) NO production in the primary cultured coelomocytes after Vibrio splendidus, Vibrio Parahaemolyticus and Escherichia Coli infection. (B) arginase activity in the primary cultured coelomocytes after Vibrio splendidus, Vibrio Parahaemolyticus and Escherichia Coli infection. Five biological replicates were performed in the experiment and the obtained data were expressed as the mean ± SD (n = 5).
Discussion
As a common substrate for a number of NO, urea, agmatine and polyamine biosynthetic pathways, arginine metabolism plays an irreplaceable role in cardiovascular function, neurotransmission, cell proliferation and immunity4,6,47. Today, the interaction between metabolism and the immune response is regulated by various arginine metabolic enzymes and constitutes an extremely intriguing area in vertebrates research. However, scarce information is available concerning the modulation of arginine metabolic pathways involved in the immune defense of invertebrates, particularly in non-model invertebrates. In this paper, we first focused on the metabolite of arginine with its key downstream molecules involved in different arginine metabolic pathways of sea cucumbers and their competitive roles in responding to the immune defense, in order to understand the crosstalk between the immune response and arginine metabolism in invertebrates.
Three typical members of AjNOS, Ajarginase and Ajagmatinase from representative arginine metabolic pathways were first isolated and characterized from the sea cucumber A. japonicus. Our results showed that the three genes were ubiquitously expressed in all examined tissues (Fig. 1), suggesting that they might be involved in versatile physiological processes28,39,48. The mRNA level of AjNOS was highly expressed in coelomocytes (4.43-fold), the respiratory tree (9.58-fold) and tentacles (11.82-fold) and was expressed most strongly in the intestine (14.95-fold). The tentacle, an important site of entry for microorganisms, especially Gram-negative pathogenic bacteria49, exhibited greater expression of AjNOS, suggesting that it plays important roles in defending against invading bacteria. Notably, the expression levels of Ajarginase and Ajagmatinase displayed almost completely opposite expression profiles compared with the AjNOS transcript, which was expressed at lower levels in other four tissues. These tissues expression profiles revealed that AjNOS might compete with Ajarginase, Ajagmatinase or both in the arginine metabolic pathways of sea cucumbers.
In most studies, haemocytes and the intestine are the most important immune tissues that play key roles in immune responses and have been used to investigate the fluctuation of immune-related genes50,51. Therefore, we were interested in the expression pattern of AjNOS, Ajarginase and Ajagmatinase mRNAs in A. japonicus coelomocytes and the intestine in response to bacterial infection in vivo and LPS challenge in vitro. The AjNOS transcript of coelomocytes was expressed significantly at 24 h and the expression level peaked at 96 h after V. splendidus stimulation compared with the control (Fig. 2). At the same time, we found that the mRNA expressions of Ajarginase and Ajagmatinase were both significantly inhibited at most detected time points of AjNOS induction. Similar results were also found in intestinal tissue, although the expression of Ajagmatinase was up-regulated 1.67-fold (P < 0.05) at 6 h after V. splendidus infection and its expression gradually decreased at the later time points. Consistently, the mRNA level of AjNOS in LPS challenge primary cells was also gradually up-regulated and reached its highest level at 24 h until the end of experiment. The Ajarginase transcript still showed opposite trends of expression to those of AjNOS, although the Ajagmatinase transcript was not significantly changed. The expression of the three genes in coelomocytes and the intestine changed rapidly and dynamically in response to the injection of V. splendidus or LPS, suggesting that they play important roles in sea cucumbers′ defense against pathogen infection. In vertebrates, iNOS could be induced by a variety of immune cells to synthesize high amounts of NO under immune defense10,17. We speculated that only one NOS isoform in A. japonicus exists, and it might possess multiple functions as a primordial NOS52. As an important cell signaling molecule, NO is often a major cytoprotective agent and controls the fate of pathogens in the immune system22,53,54. The generation of NO from arginine by NOSs is necessary for host cells to kill invading pathogens. On the contrary, the conversion of arginine to ornithine and urea in order to promote polyamine biosynthesis through the arginase pathway or alternative pathways via agmatinase can lead to the disruption of NO production of the host and enhance the survival of the bacteria40,55,56. Moreover, NG-hydroxy-L-arginine (NOHA), an intermediate in NOS, can accumulate sufficiently in iNOS-expressing cells, and it is a potent natural inhibitor of both arginase and arginase activity57,58. Based on a previous study, we surmised that the hydrolysis of increased arginine in different tissues of the sea cucumber was controlled by AjNOS under acute stress. Switching the immune response to the AjNOS reaction was required to reduce the bacteria infection.
To directly test the involvement of the arginine metabolic pathways in the sea cucumber, we used RNA interference technology to further confirm their competitive roles in arginine metabolism. siRNA-mediating RNAi is a common method of functional validation in many invertebrates in vitro, which has proven to be feasible and effective due to complex interplay of genetic and environmental influences in individual animals. In cultured cells, however, it is sometimes not stable in vitro and it does not approach stability to in vivo. Therefore, three molecules were blocked by transfecting specific siRNAs in vivo and in vitro, respectively. Our results indicated that the inhibition of Ajarginase or Ajagmatinase in vivo and in vitro, both resulted in increased NO production by induced AjNOS (Figs 4 and 5). Whereas, the expression profiles of Ajarginase and Ajagmatinase were up-regulated after AjNOS knock-down both in vivo and in vitro, accompanied by suppressed NO generation. Wijnands13 study demonstrated that arginase-I deficiency in murine endothelial cells and macrophages resulted in increased NO production by NOS during endotoxemia. In our case, the enhanced NO production in Ajarginase or Ajagmatinase inhibited by specific siRNAs was largely derived from AjNOS. Moreover, the arginase activity, a key parameter in the polyamine biosynthesis pathway and necessary for pathogen survival59,60, was significantly decreased after Ajarginase or Ajagmatinase knock-down and sharply increased after AjNOS silencing both in vivo and in vitro. There is strong evidence that constitutive levels of arginase activity generated from arginase in endothelial cells limit NOS and NO production61,62, which was in accordance with our results after AjNOS silencing. Arginase and agmatinase release urea and ornithine and putrescine, respectively, which differ from each other only by the presence of substrates. It is clear that agmatinase catalyze the hydrolysis of agmatine to urea and putrescine, participating in an alternative pathway for polyamine biosynthesis, while functioning as arginase35,63,64. Undoubtedly, arginase and NOS compete with each other in using arginine as a common substrate65,66. In our study, Ajagmatinase might also modulate arginase activity in the alternative pathway by competing with arginine and AjNOS. The findings shown here identify that AjNOS not only compete with Ajarginase but also with Ajagmatinase in arginine metabolism of the sea cucumber. This is first evidence that the alternative pathway modulated by agmatinase also plays an important role in arginine metabolism. In addition, we found that the Ajagmatinase transcript was suppressed after Ajarginase knock-down, and the Ajarginase mRNA level also dropped after Ajagmatinase interference both in vivo and in vitro. These results showed that both Ajarginase and Ajagmatinase need each other in arginine metabolism when competing with AjNOS.
To better understand the substrate competition among different arginine metabolic pathways in invertebrates responding to immune defense, we examined NO production and arginase activity from primary sea cucumber cells co-incubated with pathogens or non-pathogens at different doses and different time points. Our findings revealed that loss of arginase activity resulted in NO production, which was generally dependent on the MOI employed and time points of infection with V. splendidus and V. parahaemolyticus, especially for V. splendidus (Fig. 6). Although, primary cells challenged with non-pathogen E. coli showed NO production was significantly increased at 24 h with different MOIs followed by decreased arginase activity, which indicated that the increased NO was also able to kill non-pathogen in immune response. At an MOI of 10 after 12 h, NO production was significantly induced by AjNOS in order to kill the invading V. splendidus or V. parahaemolyticus. However, high concentrations of NO had a stronger cytotoxicity during the immune response; it could not only eliminate the intrusive pathogens but also harm to normal host cells67,68. Thus, the NO concentration was recovered to the original level at 24 h, which was essential for the maintenance of immune homeostasis of sea cucumber primary cells. At an MOI of 100, with the arginase activity increasing both with V. splendidus or V. parahaemolyticus infection, we speculated that metabolism through the arginase pathway and alternative pathway was more dominant than the NOS pathway, which might favor bacterial invasion. Most studies demonstrated that polyamines generated from the arginase or Agmatinase pathway inhibited NO production and supported bacterial growth30,31. However, host arginase activity not only promoted the spread of pathogens69, but more importantly, pathogen could also use its own arginase to compete with L-arginine from the host70. Therefore, at an MOI of 100, we speculated that the V. splendidus or V. parahaemolyticus that escaped the immune response might directly use host arginine, leading to polyamines biosynthesis pathway with increased arginase activity.
In summary, the present study first depicted the different arginine metabolic pathways in sea cucumbers, and the arginine metabolic enzymes that modulate NO production and arginase activity in the immune response were schematically presented (Fig. 7). The cationic amino acid transporter (CAT) mediated L-arginine transport4. The most exciting findings in this field originated from our studies indicating that AjNOS not only competes with Ajarginase but also with Ajagmatinase in the arginine metabolism of sea cucumbers. Moreover, the substrate competition among AjNOS, Ajarginase and Ajagmatinase to allocate arginine convert in the generation of NO and arginase activity was generally dependent on the dose of bacteria and the time course of infection. In our case, the pathogens whether directly use host arginine to promote urea release and polyamine biosynthesis will be confirmed in next work.
Figure 7. Schematic representation of the arginine metabolic pathways of host-pathogen interaction.
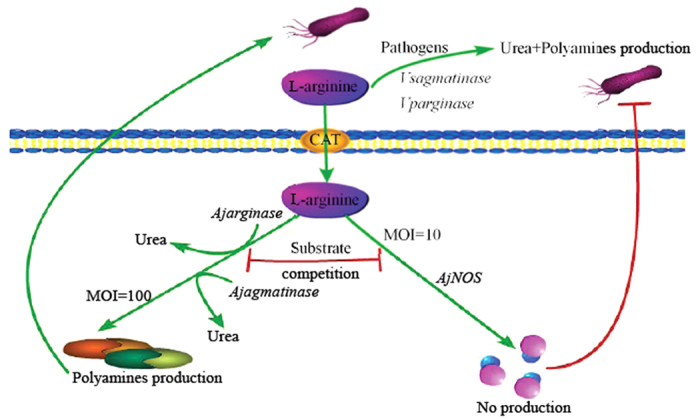
CAT was short from cationic amino acid transporter and mediated arginine transport. The extracellular urea released by pathogens (Vsagmatinase or Vparginase). Ajarginase and Ajagmatinase transcribed and translated and generated into urea. AjNOS transcribed and translated and generated into NO.
Methods
Animals and challenge experiments
Sea cucumbers A. japonicus (weight 125 ± 15 g) were collected from Bowang Aquaculture Company (Ningbo, China) and reared in 30 L aerated natural seawater (salinity 28, temperature 16 °C) for 3 days. For the challenge experiments, sea cucumbers were randomly divided into four tanks, each containing twenty individuals. The three experimental groups were infected with live V. splendidus at a final concentration of 107 CFU mL−1. The infection dose and sampling points were determined by immune genes expression analysis. Five individuals were randomly sampled at 6, 24, 48, 72 and 96 h post infection. The remaining twenty untreated sea cucumber served as control group and were collected at 0 h. Coelomic fluids were collected through a 300 Mesh CellCribble and then centrifuged at 800 × g for 5 min to harvest the coelomocytes along with the intestine for time-course expression analysis. For spatial expression analysis, coelomocytes, intestine tissue and three other tissues, including muscle, tentacle and respiratory trees, were collected from control individuals using sterilized scissors and tweezers. All tissues (approx. 100 mg wet weight) were ground into powder in liquid nitrogen using a mortar and a pestle. We performed 5 replicates in the experimental group as well as the control group and all samples were stored at −80 °C for further analysis.
Rapid application of cDNA ends (RACE)
Partial sequences of NOS, arginase and agmatinase genes were generated by screening A. japonicus transcriptome database71. Gene-specific primers for three genes (Table 1) were designed based on the acquired unigenes and the full-length cDNA sequences were subsequently cloned using the 3′, 5′-Full RACE Kit (TaKaRa) following the manufacturer’s instructions. The desired PCR products were purified and then cloned into the pMD18-T simple vector (TaKaRa). Three positive clones for each product were sequenced at Sangon (Shanghai, China). The sequences were overlapped, verified, and subjected to cluster analysis.
Table 1. Primers used in this study.
| Primer Name | Primer Sequence (5′–3′) | Used for |
|---|---|---|
| AjNOS 3-1 | CAGGTTTTTGATGCACGAAATGC | 3′ RACE |
| AjNOS 3-2 | CGCCAATCTCTGGAAGCATCACT | |
| AjNOS 3-3 | CGAGACATTCTGCGTGGGTAAGG | |
| AjNOS 3-4 | CTGACGCTGCTCGGAAAAGGTAG | |
| AjNOS 3-5 | AGCGACCAAGGCAAAATGAGTAT | |
| AjNOS 5-1 | GCACCAAAGATGAGTTCCATTTC | 5′ RACE |
| AjNOS 5-2 | ACTCGCTCTCCTTTCCTTCTACC | |
| AjNOS 5-3 | CCGTAATTCCATGTGTCTCGCTCT | |
| AjNOS 5-4 | TCGGCTAATGTGGTCCTCTGGTT | |
| AjNOS 5-5 | GCCGTTTGAGGAGCCTTATTGAG | |
| Agarginase 3-1 | ACAAGCACGGATTACCAGAAGTC | 3′ RACE |
| Agarginase 3-2 | ACACCAAGCACAGGCACAAGAGT | |
| Agarginase 5-1 | GATTAGGTCTCCTTCCCTTATGG | 5′ RACE |
| Agarginase 5-2 | TGGACTGAAAAATGGGTGACAAC | |
| Ajagmatinase 3-1 | ATGGAATCATCAAAGGGTCTGGA | 3′ RACE |
| Ajagmatinase 3-2 | TTGGTCCGAGGCAAATAAGAACT | |
| Ajagmatinase 5-1 | AATGTCGGCAACTCGTAAGGATG | 5′ RACE |
| Ajagmatinase 5-2 | ATTCCAACAAAGCAGGCATCCAG | |
| AjNOS F | GTAGAAGGAAAGGAGAGCGAGTC | Real-time PCR |
| AjNOS R | CATCGTGTCTCGTCGCATAGTGT | |
| Ajarginase F | AAGCGTTGGGATTCTCGGTGTG | Real-time PCR |
| Ajarginase R | TGGGAGTTCTTCACGAGAGGTTG | |
| Ajagmatinase F | CCTTACGAGTTGCCGACATTGGT | Real-time PCR |
| Ajagmatinase R | CTCGTCAAATGCCCTACGGAATG | |
| Ajβ-actin F | CCATTCAACCCTAAAGCCAACA | Real-time PCR |
| Ajβ-actin R | ACACACCGTCTCCTGAGTCCAT | |
| siRNA interference sequences | AjNOS interference | |
| Sense 1 | GCGACGAGACACGAUGUUUTT | |
| Anti-sense 1 | AAACAUCGUGUCUCGUCGCTT | |
| Sense 2 | GCUGCAGUUCACCGGUAUUTT | |
| Anti-sense 2 | AAUACCGGUGAACUGCAGCTT | |
| Sense 1 | GGACGAUUGUGCCGAUCAATT | Ajarginase interference |
| Anti-sense 1 | UUGAUCGGCACAAUCGUCCTT | |
| Sense 2 | GCGAGAGUACAUGCCACAATT | |
| Anti-sense 2 | UUGUGGCAUGUACUCUCGCTT | |
| Sense 1 | GCGGAUUCAUCGGACGAAATT | Ajagmatinase interference |
| Anti-sense 1 | UUUCGUCCGAUGAAUCCGCTT | |
| Sense 2 | CCAUCAGUGGAUUAGAUAUTT | |
| Anti-sense 2 | AUAUCUAAUCCACUGAUGGTT | |
| Sense | UUCUCCGAACGUGUCACGUTT | Negative control (NC) interference |
| Anti-sense | ACGUGACACGUUCGGAGAATT |
Sequence analysis
Sequences homology were obtained using BLAST program at National Centre for Biotechnology Information (http://www.ncbi.nlm.nih.gov/blast) and the deduced amino acid sequences of AjNOS, Ajarginase and Ajagmatinase were analyzed with the expert protein analysis system (http://www.expasy.org/). The molecular mass (MM) and theoretical pI of the protein were calculated based upon its deduced amino acids by the ProtParam tool (http://www.expasy.ch/tools/protparam.html). Domain in these amino acid sequences were detected using the simple modular architecture research tool (SMART) program (http://www.smart.emblheidelbergde/). Multiple alignments analysis of each protein were performed using the ClustalW2 Multiple Alignment program (http://www.ebi.ac.uk/clustalw/) and the Multiple Align Show program (http://www.bio-soft.net/sms/index.html). Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 4.0 program.
Quantitative real-time PCR analysis of AjNOS, Ajarginase and Ajagmatinase mRNAs expression
The tissue distribution and time-course expression of AjNOS, Ajarginase and Ajagmatinase were performed using a Rotor-Gene 6000 real-time PCR detection system. Total RNA was isolated from coelomocytes and other tissues using Trizol (Invitrogen), and cDNA was synthesized using the Primescript™ II 1st cDNA Synthesis Kit (Takara). The employed primers are listed in Table 1. Amplifications were carried out in a 20 μL reaction volume, containing 8 μL of the 1:100 diluted cDNA, 1 μL of each of the primers, and 10 μL of SYBR Green Mix (Takara). The reaction mixture was incubated for 5 min at 95 °C, followed by 40 amplification cycles of 15 s at 95 °C, 20 s at 60 °C, and 20 s at 72 °C. To maintain consistency, the baseline was set automatically by the software. The 2−ΔΔCT method was used to analyze the relative expression level of the candidate genes72, and the value obtained denoted the n-fold difference relative to the calibrator. Quantitative data were expressed as the mean ± standard deviation (SD) of five biological replicates. One-way (ANOVA) was applied to discern significant differences between control and experimental groups. Any significant differences relative to the control for each time point were indicated with an asterisk at P < 0.05 and two asterisks at P < 0.01.
Temporal expression profiles of AjNOS, Ajarginase and Ajagmatinase in LPS-exposed primary coelomocytes
Sea cucumbers (weight 125 ± 15 g) were dissected with sterilized scissors on ice as described73,74. In brief, the coelomic fluids were filtered through a 300 Mesh CellCribble to remove large tissue debris, mixed with the anticoagulant solution (0.02 M EGTA, 0.48 M NaCl, 0.019 M KCl, 0.068 M Tri-HCl, pH7.6) in a 1:1(V:V) ratio, and then centrifuged at 800 × g, 16 °C for 10 min. The harvested cells were washed twice with isotonic buffer (0.001 M EGTA, 0.53 M NaCl, 0.01 M Tris-HCl, pH7.6) and re-suspended in the Leiboviz’s L-15 cell culture medium (Invitrogen, USA) containing penicillin (100 U mL−1) and streptomycin sulfate (100 mg mL−1). The cells were diluted to 106 cells mL−1 and transferred into a 24 well culture microplates and incubated in 16 °C for 12 h prior to lipopolysaccharides (Sigma, USA) exposure. For the LPS challenge, the cells were stimulated with 10 μg mL−1 LPS for 3, 6, 12 and 24 h. The untreated cells served as control and were sampled at 0 h. The cells were collected and dissolved in Trizol and used for gene expression analysis. qPCR and statistical analysis were conducted as described above.
Functional validation of AjNOS, Ajarginase and Ajagmatinase in primary coelomocytes, by siRNA
Small interfering RNAs (siRNA) targeting AjNOS, Ajarginase and Ajagmatinase were designed and synthesized by GenePharma (Shanghai, China). Another siRNA (negative control, NC) that did not target any of the genes in sea cucumber transcriptome data served as the control. Two specific siRNAs were designed for each gene for RNA interference. The detailed sequences are shown in Table 1. These siRNAs were then dissolved into RNase-free water to obtain a working solution of 20 μM. For RNA interference, 2 μL siRNAs (80 nM) of AjNOS, Ajarginase and Ajagmatinase (Table 1, Genepharma, China) were mixed with 1 μL siRNA-Mate (GenePharma), and then transfected into 500 μL primary cultured cells in each well. The primary cells with non-targeted double-strand siRNA (Table 1) served as a control group. At 24 h post-transfection, half of the cells were harvested, dissolved in Trizol, and used for a gene silencing efficiency assay. The rest of the cells were collected for NO production and arginase activity analysis.
Functional validation of AjNOS, Ajarginase and Ajagmatinase in vivo by siRNA
Sea cucumbers A. japonicus (weight: 82 ± 10 g) were used for in vivo RNAi experiments. Briefly, the six identical siRNA sequences of AjNOS, Ajarginase, and Ajagmatinase (Table 1) with extra 2’ Ome modification were designed for the in vivo assay and synthesized by GenePharma (Shanghai, China). Each siRNA and a negative control were dissolved in RNase-free water to obtain a working solution of 20 μM. We mixed 10 μL of each siRNA (400 nM) or a negative conrol with 10 μL of transfection reagent (Beyotime biotechology, China) and 80 μL of phosphate buffered solution (PBS) at pH 7.6 to serve as the transfection solution. Sea cucumbers were injected with 100 μL of transfection solution via tentacles. After 24 h injection, the treated and control coelomocytes were collected for expression analysis, NO production and arginase activity analysis. The assays described above were biologically repeated five times.
Dose-dependent expression profiles of arginine in pathogen- or non-pathogen-challenged coelomocytes in vitro
A. japonicus primary cells were cultured in L-15 cell culture medium (Invitrogen, USA) containing penicillin (100 U mL−1) and streptomycin sulfate (100 mg mL−1) as described above. After 12 h of culturing for recovery, the culture medium was discarded, and the cells were washed two times in PBS and then replaced with antibiotic-free L-15 with live V. splendidus, V. parahaemolyticus, and E. coli added at appropriate multiplicites of infection (MOIs) of 10 and 100, respectively. The cultured primary cells operated as before without bacterial co-incubation served as the control. The primary cells with each bacterial co-incubation and control group were incubated at 16 °C for 12 and 24 h. Finally, the culture medium was discarded, and the cells were washed two times in PBS and collected for the detection of NO production and arginase activity.
Measurement of NO production and arginase activity
NO production in A. japonicus primary cells from the stimulation and control groups were analyzed with an NO colorimetric assay kit (Jiancheng, Nanjing, China). Absorbance was measured at OD550 nm using a microplate reader (Thermo Scientific). The protein concentration of cells was measured using the BCA Protein Assay Kit (Sangon, China). The NO content was expressed as μmol/gprot.
Arginase activity was measured via a colorimetric assay for the detection of urea production from L-arginine as described previously75,76 and with minor modifications. Approximately 105 cells were mixed with 50 μL cell lysis buffer (Beyotime biotechology, China) and stirred for 30 min at room temperature. After the cells were lysed, the cells were centrifuged at 12,000 × g for 5 min and the supernatant was transferred to a centrifuge tube. Approximately 25 μL of 10 mM MnC12 was added and the mixture was activated for 10 min at 56 °C. The mixture was incubated with 50 μL L-arginine (0.5M, pH 9.7) for one hour at 37 °C to hydrolyze the L-arginine. The hydrolysis reaction was stopped with an acid mixture containing H2SO4, H3PO4 and H2O (1:3:7) and the mixture was then heated at 100 °C with 25 μL of α-isonitrosopropiophenone (9% α-ISPF in absolute ethyl alcohol) for 45 min. The samples were kept in the dark at room temperature for 10 min, and the absorbance was measured at 540 nm in a microplate reader. One-way ANOVA was applied to discern significant differences between control and experimental groups. Any significant differences relative to the control for each time point were indicated by an asterisk at P < 0.05 and two asterisks at P < 0.01.
Additional Information
How to cite this article: Yina, S. et al. The first description of complete invertebrate arginine metabolism pathways implies dose-dependent pathogen regulation in Apostichopus japonicus. Sci. Rep. 6, 23783; doi: 10.1038/srep23783 (2016).
Supplementary Material
Acknowledgments
This work was financially supported through NSFC (31522059, 41576139), the Zhejiang Provincial Natural Science Foundation of China (LR14C190001), the Outstanding (Postgraduate) Dissertation Growth Foundation of Ningbo University (PY2014001), Leading and Top-notch Talent Project of Ningbo, Collaborative Innovation Center for Zhejiang Marine High-efficiency and Healthy Aquaculture, and the K.C. Wong Magna Fund at Ningbo University.
Footnotes
Author Contributions S.Y. performed the whole experiment and wrote the manuscript. L.C. designed the experiments, wrote and revised the manuscript, and contributed reagents. Z.W. contributed reagents/materials/analysis tools. W.Z. discussed the project and analyzed data; L.Z. discussed the project and analyzed data. All authors contributed to the editing of the manuscript.
References
- Ouzounis C. A. & Kyrpides N. C. On the evolution of arginases and related enzymes. J Mol Evol 39, 101–104 (1994). [DOI] [PubMed] [Google Scholar]
- Li P., Yin Y., Li D., Kim S. W. & Wu G. Amino acids and immune function. Brit J Nutr 98, 237–252 (2007). [DOI] [PubMed] [Google Scholar]
- Morris S. M. Jr. Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol 15, 922–930 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorin J. et al. Arginine and nitric oxide synthase: regulatory mechanisms and cardiovascular aspects. Moi Nutr Food Res 58, 101–116 (2014). [DOI] [PubMed] [Google Scholar]
- King N. E., Rothenberg M. E. & Zimmermann N. Arginine in asthma and lung inflammation. J Nutr 134, 2830S–2836S (2004). [DOI] [PubMed] [Google Scholar]
- Nieves C. & Langkamp-Henken B. Arginine and immunity: a unique perspective. Biomed Pharmacother 56, 471–482 (2002). [DOI] [PubMed] [Google Scholar]
- Das P., Lahiri A., Lahiri A. & Chakravortty D. Modulation of the Arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator. PLos Pathog 6, e1000899 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanasen N. & Soong L. L-arginine metabolism and its impact on host immunity against Leishmania infection. Immunol Res 41, 15–25 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic P. J., Zeh H. J. III & Ochoa J. B. Arginine and immunity. J Nutr 137, 1681S–1686S (2007). [DOI] [PubMed] [Google Scholar]
- Alderton W. K., Cooper C. E. & Knowles R. G. Nitric oxide synthases: structure, function and inhibition. Biochem J 357, 593–615 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. & Stuehr D. J. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol 57, 707–736 (1995). [DOI] [PubMed] [Google Scholar]
- Bansal V. & Ochoa J. B. Arginine availability, arginase, and the immune response. Curr Opin Clin Nutr Metab Care 6, 223–228 (2003). [DOI] [PubMed] [Google Scholar]
- Wijnands K. A. P. et al. Arginase-1 deficiency regulates arginine concentrations and NOS2-mediated NO production during endotoxemia. PLos ONE 9, e86135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Soud H. M. & Stuehr D. J. Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci USA 90, 10769–10772 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller D. A. & Billiar T. R. Molecular biology of nitric oxide synthases. Cancer Metastasis Rev 17, 7–23 (1998). [DOI] [PubMed] [Google Scholar]
- Cho H. J. et al. Calmodulin is a subunit of nitric-oxide synthase from Macrophages. J Exp Med 176, 599–604 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C. The multiplex function of nitric oxide in (auto) immunity. J Exp Med 187, 1361–1365 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F. C. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest 99, 2818–2825 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P. Nitric oxide and immune response. Indian J Biochem Biophys 44, 310–319 (2007). [PubMed] [Google Scholar]
- Yao J. et al. Expression of nitric oxide synthase (NOS) genes in channel catfish is highly regulated and time dependent after bacterial challenges. Dev Comp Immunol 45, 74–86 (2014). [DOI] [PubMed] [Google Scholar]
- Weiske J. & Wiesner A. Stimulation of NO synthase activity in the immune-competent lepidopteran Estigmene acraea hemocyte line. Nitric Oxide 3, 123–131 (1999). [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M. J. & Higgs E. A. Biosynthesis of nitric oxide fromL-arginine: a pathway for the regulation of cell function and communication. Biochem Pharmacol 38, 1709–1715 (1989). [DOI] [PubMed] [Google Scholar]
- Nakane M., Schmidt H. H. H. W., Pollock J. S., Forstermann U. & Murad F. Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett 31, 175–180 (1993). [DOI] [PubMed] [Google Scholar]
- Wang T., Ward M., Grabowski P. & Secombes C. J. Molecular cloning, gene organization and expression of rainbow trout (Oncorhynchus mykiss) inducible nitric oxide synthase (iNOS) gene. Biochem J 358, 747–755 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulski M. & Tully T. Molecular and biochemical characterization of dNOS: a Drosophila Ca2+/calmodulin-dependent nitric oxide synthase. Proc Natl Acad Sci USA 92, 9072–9076 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C., Ji P., Wang Z., Li F. & Xiang J. Molecular cloning and expression of NOS in shrimp. Litopenaeus vannamei. Fish Shellfish Immunol 28, 453–460 (2010). [DOI] [PubMed] [Google Scholar]
- Chen T. et al. Nitric oxide as an antimicrobial molecule against Vibrio harveyi infection in the hepatopancreas of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 42, 114–120 (2014). [DOI] [PubMed] [Google Scholar]
- Jiang Q. et al. The immunomodulation of inducible nitric oxide in scallop Chlamys farreri. Fish Shellfish Immunol 34, 100–108 (2013). [DOI] [PubMed] [Google Scholar]
- Berkowitz D. E. et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108, 2000–2006 (2003). [DOI] [PubMed] [Google Scholar]
- Chang C. I., Liao J. C. & Kuo L. Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer Res 61, 1100–1106 (2001). [PubMed] [Google Scholar]
- Ghosh S. et al. Arginine-induced germ tube formation in Candida albicans is essential for escape from murine macrophage line RAW 264.7. Infect Immun 77, 1596–1605 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussiere F. I. et al. Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric-oxide synthase translation. J Biol Chem 280, 2409–2412 (2005). [DOI] [PubMed] [Google Scholar]
- Borlace G. N. et al. A role for altered phagosome maturation in the long-term persistence of Helicobacter pyloriinfection. Am J Physiol Gastrointest Liver Physiol 303, G169–G179 (2012). [DOI] [PubMed] [Google Scholar]
- Kim J. H. et al. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol 107, 1249–1257 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn H. J. et al. Crystal structure of Agmatinase reveals structural conservation and inhibition mechanism of the ureohydrolase superfamily. J Biol Chem 279, 50505–50513 (2004). [DOI] [PubMed] [Google Scholar]
- Wang X. et al. Arginine decarboxylase and Agmatinase: an alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol Reprod 90, 1–15 (2014). [DOI] [PubMed] [Google Scholar]
- Perozich J., Hempel J. & Morris S. M. Jr. Roles of conserved residues in the arginase family. Bioch Bioph Acta 1382, 23–37 (1998). [DOI] [PubMed] [Google Scholar]
- Goda S., Sakuraba H., Kawarabayasi Y. & Ohshima T. The first archaeal agmatinase from anaerobic hyperthermophilic archaeon Pyrococcus horikoshii: cloning, expression, and characterization. Bioch Bioph Acta 1748, 110–115 (2008). [DOI] [PubMed] [Google Scholar]
- Iyer R. K., Kim H. K., Tsoa R. W., Grody W. W. & Cederbaum S. D. Cloning and characterization of human Agmatinase. Mol Genet Metab 75, 209–218 (2002). [DOI] [PubMed] [Google Scholar]
- Galea E., Regunathan S., Eliopoulos V., Feinstein D. L. & Reis D. J. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem J 316, 247–249 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demady D. R., Jianmongkol S., Vuletich J. L., Bender A. T. & Osawa Y. Agmatine enhances the NADPH oxidase activity of neuronal NO synthase and leads to oxidative inactivation of the enzyme. Mol Pharmacol 59, 24–29 (2001). [DOI] [PubMed] [Google Scholar]
- Satriano J. et al. Agmatine suppresses proliferation by frameshift induction of antizyme and attenuation of cellular polyamine levels. J Biol Chem 273, 15313–15316 (1998). [DOI] [PubMed] [Google Scholar]
- Deng H. et al. Isolation and pathogenicity of pathogens from skin ulceration disease and viscera ejection syndrome of the sea cucumber Apostichopus japonicus. Aquaculture 287, 18–27 (2009). [Google Scholar]
- Liu H. et al. Identification of the pathogens associated with skin ulceration and peristome tumescence in cultured sea cucumbers Apostichopus japonicus (Selenka). J Invertebr Pathol 105, 236–242 (2010). [DOI] [PubMed] [Google Scholar]
- Jiang Y. Characte rization of antimicrobial resistance of Vibrio parahaemolyticus from cultured sea cucumbers (Apostichopus japonicas). Lett Appl Microbiol 59, 147–154 (2014). [DOI] [PubMed] [Google Scholar]
- Shao Y. et al. Divergent metabolic responses of Apostichopus japonicus suffered from skin ulceration syndrome and pathogen challenge. J Agric Food Chem 61, 10766–10771 (2013). [DOI] [PubMed] [Google Scholar]
- Jiang Q. et al. Mutual modulation between norepinephrine and nitric oxide in haemocytes during the mollusc immune response. Sci Rep 4, 6963 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerink M., Savelkoul H. F. J. & Wiegertjes G. F. Evolutionary conservation of alternative activation of macrophages: structural and functional characterization of arginase 1 and 2 in carp (Cyprinus carpio L.). Mol Immunol 43, 1116–1128 (2006). [DOI] [PubMed] [Google Scholar]
- Graham E. R. & Thompson J. T. Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J Exp Mar Biol Ecol 368, 22–29 (2009). [Google Scholar]
- Zhang L. et al. Cloning and characterization of allograft inflammatory factor-1 (AIF-1) from manila clam Venerupis philippinarum. Fish Shellfish Immunol 30, 148–53 (2011). [DOI] [PubMed] [Google Scholar]
- Losada A. P. et al. Quantitative and qualitative evaluation of iNOS expression in turbot (Psetta maxima) infected with Enteromyxum scophthalmi. Fish Shellfish Immunol 32, 243–248 (2012). [DOI] [PubMed] [Google Scholar]
- Andreakis N. D. et al. Evolution of the nitric oxide synthase family in metazoans. Mol Biol Evol 28, 163–179 (2011). [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat Immunol 2, 907–916 (2001). [DOI] [PubMed] [Google Scholar]
- Thomas D. D. et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med 45, 18–31 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert A. P. et al. L-Arginine availability modulates local nitric oxide production and parasite killing in experimental trypanosomiasis. Infect Immun 68, 4653–4657 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auguet M., Viossat I., Marin J. G. & Chabrier P. E. Selective inhibition of inducible nitric oxide synthase by agmatine. J Pharmacol 69, 285–287 (1995). [DOI] [PubMed] [Google Scholar]
- Daghigh F., Fukuto J. & Ash D. E. Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications for the regulation of nitric oxide biosynthesis by arginase. Biochem Biophys Res Commun 202, 174–180 (1994). [DOI] [PubMed] [Google Scholar]
- Buga G. M. et al. Arginase activity in endothelial cells: inhibition by NG-hydroxyarginine during high-output nitric oxide production. Am J Physiol 271, H1988–H1998 (1996). [DOI] [PubMed] [Google Scholar]
- Hesse M. et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by Type 1/Type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol 2001, 167, 6533–6544 (2001). [DOI] [PubMed] [Google Scholar]
- Yu H. et al. Expression of arginase isozymes in mouse brain. J Neurosci Res 66, 406–422 (2001). [DOI] [PubMed] [Google Scholar]
- Zhang C., Hein T. W., Wang W., Chang C. I. & Kuo L. Constitutive expression of arginase in microvascular endothelial cells counter-acts nitric oxide-mediated vasodilatory function. FASEB J 15, 1264–1266 (2001). [DOI] [PubMed] [Google Scholar]
- Lim H. K. et al. Mitochondrial arginase II constrains endothelial NOS-3 activity. Am J Physiol Heart Circ Physiol 293, H3317–H3324 (2007). [DOI] [PubMed] [Google Scholar]
- Wiesinger H. Arginine metabolism and the synthesis of nitric oxide in the nervous system. Prog Neurobiol 64, 365–391 (2001). [DOI] [PubMed] [Google Scholar]
- Sekowska A., Danchin A. & Risler J. L. Phylogeny of related functions: the case of polyamine biosynthetic enzymes. Microbiology 146, 1815–1828 (2000). [DOI] [PubMed] [Google Scholar]
- Chang C. I., Liao J. C. & Kuo L. Arginase modulates nitric oxide production in activated macrophages. Am J Physiol 274, H342–H348 (1998). [DOI] [PubMed] [Google Scholar]
- Morris S. M. Jr. Arginine metabolism in vascular biology and disease. Vasc Med. 10, S83–87 (2005). [DOI] [PubMed] [Google Scholar]
- Hibbs J. B. Jr., Taintor R. R., Vavrin Z. & Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun 157, 87–94 (1998). [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M. & Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43, 109–142 (1991). [PubMed] [Google Scholar]
- Iniesta V. et al. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunol 24, 113–118 (2002). [DOI] [PubMed] [Google Scholar]
- Vincendeau P., Gobert A. P., Daulouede S., Moynet D. & Mossalayi M. D. Arginases in parasitic diseases. Trends Parasitol 19, 9–12 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang P. et al. De novo assembly of the sea cucumber Apostichopus japonicus hemocytes transcriptome to identify miRNA targets associated with skin ulceration syndrome. PLos ONE 8, e73506 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- Xing J., Leung M. F. & Chia F. S. Quantitative analysis of phagocytosis by amebocytes of a sea cucumber, Holothuria leucospilota. Invertebr Biol 117, 13–22 (1998). [Google Scholar]
- Gu M. et al. Immune response of sea cucumber Apostichopus japonicus coelomocytes to several immunostimulants in vitro. Aquaculture 306, 49–56 (2010). [Google Scholar]
- Corraliza I. M., Campo M. L., Soler G. & Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods 174, 231–235 (1994). [DOI] [PubMed] [Google Scholar]
- Shatanawi A., Gharaibeh M. N., Caldwell R. B. & Caldwell R. W. High glucose upregulates Arginase 1 and decreases Nitric oxide production through ATF-2 and c-Jun transcription factors. Life Sci J 11, 374–379 (2014). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.