FIGURE 1.
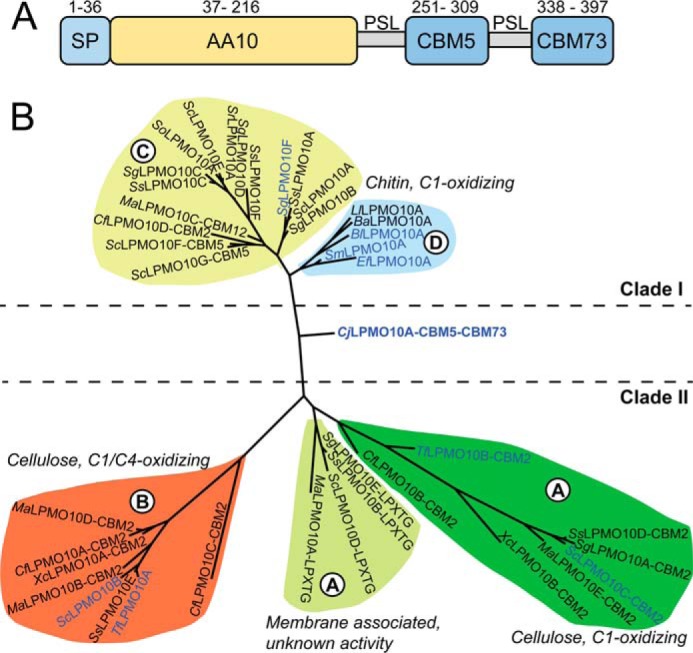
Sequence analysis of CjLPMO10A. A, domain architecture of CjLPMO10A. The full-length enzyme contains a signal peptide (SP: residues 1–36) that is cleaved off during secretion resulting in the mature enzyme that possesses an N-terminal AA10-type LPMO domain followed by a family 5 chitin-binding module (CBM5) and a C-terminal CBM classified as CBM73 (see text). The three domains are separated by two poly-serine linkers (PSL). The modules and linkers are scaled according to the number of amino acids they contain. B, phylogeny of selected LPMOs from auxiliary activities family 10 built on a previous classification by Book et al. (62), using Phylogeny.fr (64). Enzyme names include additional domains and other relevant sequence motifs, but the phylogeny is based on the catalytic domains only. The indicated clades are as defined in the study by Book et al. (62). Clade I (subclade C and D) contains chitin-oxidizing LPMOs and Clade II (subclade A and B) contains cellulose-oxidizing LPMOs as well as membrane-associated LPMOs with unknown function. Blue colored protein names represent enzymes that have been characterized.
