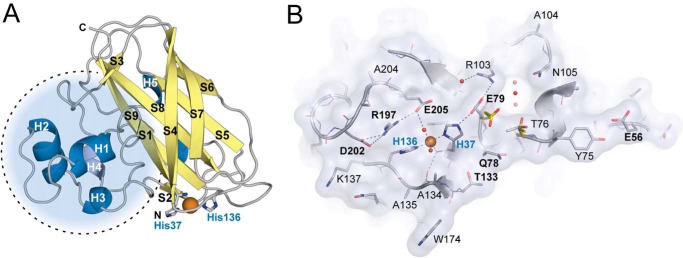
FIGURE 3.
Three-dimensional structure of CjLPMO10Acd. A, cartoon representation of the CjLPMO10Acd secondary structure. α-Helices are shown in blue, and β-strands are shown in yellow. The copper ion is shown as an orange sphere, and the coordinating histidine side chains (His-37 and His-136) are shown as gray sticks. The dotted circle shows the protrusion that includes most of the structural diversity in LPMOs. B, surface projection of the substrate binding surface in CjLPMO10Acd, chain B. The main chain is shown in cartoon representation, and side chains are shown as sticks. The copper ion and selected solvent water molecules are shown as orange and red spheres, respectively. Surface-exposed residues are labeled using the single letter amino acid code, and those discussed in the main text are marked with bold letters (E56, Q78, E79, T133, R197, D202, and E205). Glu-79 and Thr-76 show different rotamers in chain A (yellow sticks) compared with chain B and C (chain B; gray sticks).