FIGURE 3.
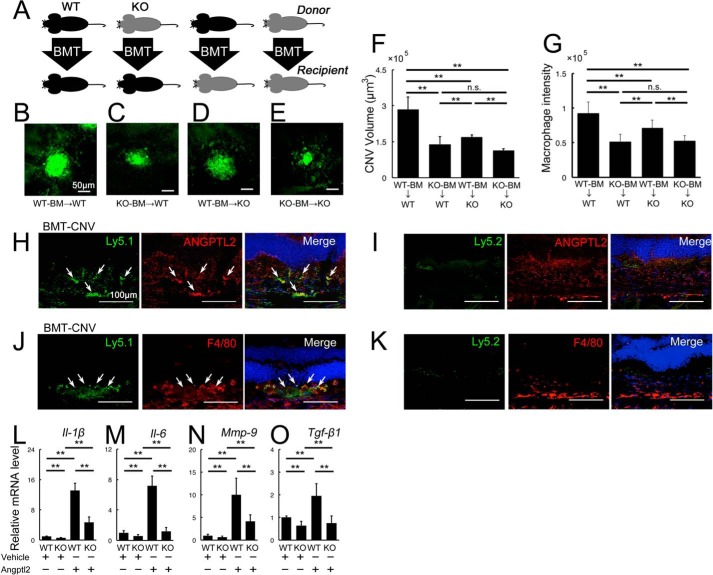
Angptl2 derived from both transplanted BMCs and the host contribute to laser-induced CNV development. A–G, BM-transplanted mice were exposed to laser photocoagulation, and the CNV volumes were measured 7 days later. A, cartoon depicting the various combinations of donor and recipient genotypes. WT is shown in black, and KO is in gray. B–F, lectin-stained CNV from BMC-transplanted mice 7 days after laser treatment. Scale bar, 50 μm. B and C, compared with WT-BMC-transplanted WT hosts (B), KO-BMC-transplanted WT hosts (C), and WT-BMC-transplanted KO hosts (D) developed CNV with reduced volumes. E, the CNV volumes were further reduced in KO-BMC-transplanted KO hosts. F, graphical representation of the average CNV volume in each group of BMC-transplanted mice. G, immunofluorescence intensity of F4/80, a macrophage marker, in the BM-transplanted mice 3 days after laser treatment. The macrophage level was comparable to the CNV volume in each group. H–K, double immunostaining of the retinal sections with the BMC markers, Ly5.1 or Ly5.2, and Angptl2, in the retinal sections of Ly5.1-positive BMC-transplanted Ly5.2-positive hosts in which Ly5.2-positive BMCs were eliminated by x-ray irradiation prior to BMT. Ly5.1-positive transplanted BMCs expressed Angptl2 (H, arrows) and F4/80 (J, arrows), but Ly5.2-positive BMCs were not observed in the lesion (I and K). L–O, real time RT-PCR analyses of inflammatory mediators in the peritoneal macrophages harvested from WT and KO mice. All the mRNAs of Il-1β (L), Il-6 (M), Mmp-9 (N), and Tgf-β1 (O) were suppressed in the macrophages of the KO mice, whereas all the mRNAs were up-regulated in response to additional recombinant Angptl2 protein in the macrophages of both WT and KO mice. KO, Angptl2 knock-out. Scale bar, 50 μm. n = 10. **, p < 0.01.