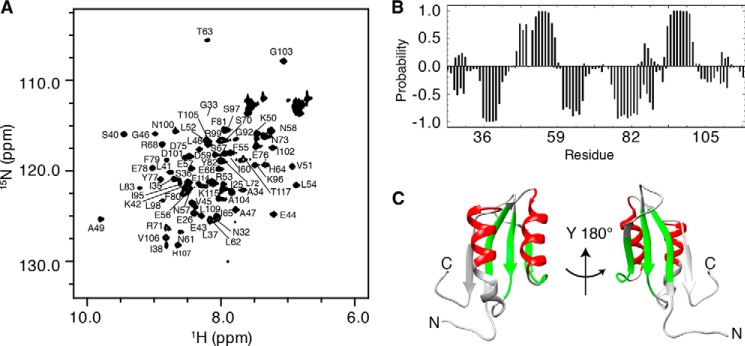
FIGURE 1.
Structural analysis of RDPheH(25–117) by NMR spectroscopy. A, two-dimensional 1H-15N HSQC spectrum of 0.48 mm RDPheH(25–117) plus 2 mm phenylalanine showing the assignments of the individual residues. Conditions are as follows: 50 mm sodium phosphate, 100 mm NaCl, 1 μm leupeptin, 1 μm pepstatin A, and 5% D2O, pH 8.0, at 298 K at a magnetic field strength of 14.1 tesla (600 MHz 1H). B, secondary structure prediction for RDPheH(25–117) in the presence of phenylalanine using PECAN (32). The probabilities of α-helices and β-strands are given as positive and negative values, respectively. C, α-helices (red) and β-strands (green) predicted in B mapped to the crystal structure of the regulatory domain of PheH (Protein Data Bank code 2PHM, residues 25–117).