FIGURE 5.
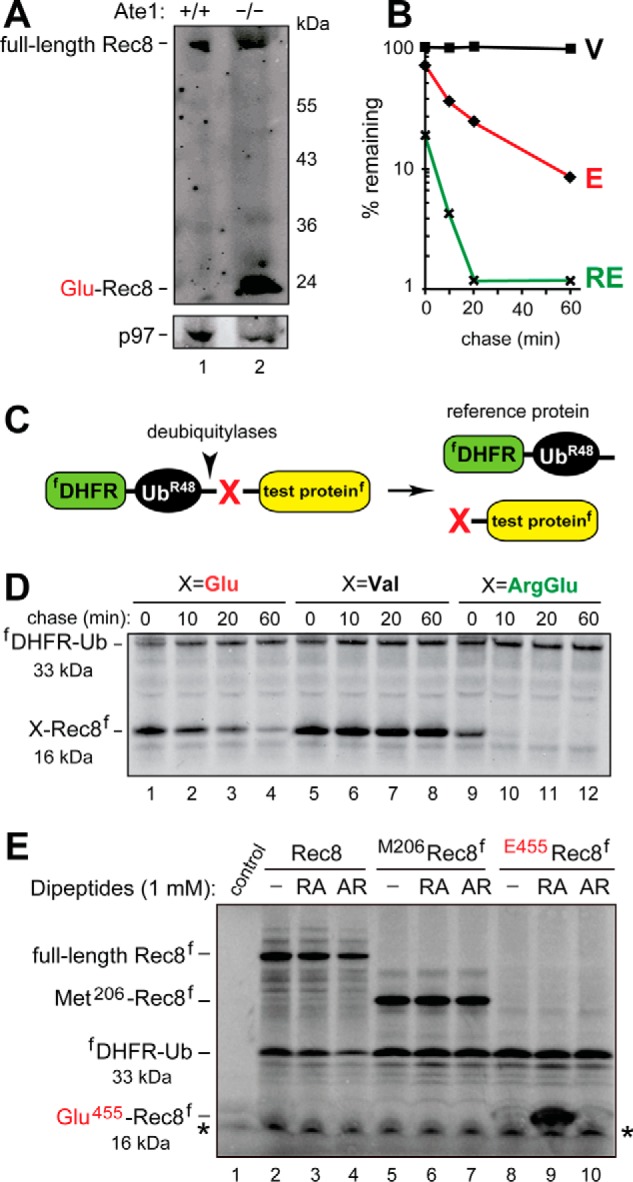
The separase-generated C-terminal fragment of Rec8 as a short-lived substrate of the Arg/N-end rule pathway. A, immunoblotting of extracts from wild-type (lane 1) versus Tnap-Ate1−/− (lane 2) mouse testes with antibody to a C-terminal region of Rec8. Note the presence of a prominent band, inferred to be the endogenous Glu455-Rec8 fragment, in Tnap-Ate1−/− but not wild-type testes. The bottom panel shows the results of control immunoblots using antibody to the unrelated p97 protein. B, quantification of data in D. C, the URT (see “Experimental Procedures”). D, lanes 1–4, 35S-pulse-chase of the C-terminally FLAG-tagged Glu455-Rec8f fragment of mouse Rec8, produced as the URT fusion fDHFR-UbR48-Glu455-Rec8f in reticulocyte extract (see “Experimental Procedures”). The bands of Glu455-Rec8f and the reference protein fDHFR-UbR48 are indicated on the left. Lanes 5–8, same as in lanes 1–4 but with the otherwise identical Val455-Rec8f, bearing N-terminal Val, which is not targeted by the Arg/N-end rule pathway. Lanes 9–12, same as in A but with Arg-Glu455-Rec8f (see “Experimental Procedures”). E, in these assays, the synthesis-deubiquitylation-degradation of URT-based fDHFR-UbR48-X-Rec8f fusions in reticulocyte extract was allowed to proceed for 1 h, followed by detection of Glu455-Rec8f, of other test proteins, and of the fDHFR-UbR48 reference protein by SDS-PAGE and immunoblotting with anti-FLAG antibody. Lane 1, control extract with the added vector plasmid (see “Experimental Procedures”). Lane 2, expression of the fDHFR-UbR48-Rec8f fusion, which is processed by deubiquitylases in the extract to yield full-length Rec8f. Lane 3, same as lane 2 but in the presence of the Arg-Ala (RA) dipeptide at 1 mm. Lane 4, same as lane 3 but in the presence of the Ala-Arg (AR) dipeptide at 1 mm. Lanes 5–7, same as lanes 2–4 but with expression of the fDHFR-UbR48-Met206-Rec8f fusion, which is processed in the extract to yield fDHFR-UbR48 and Met206-Rec8f (see “Experimental Procedures”). Lanes 8–10, same as lanes 5–7 but with expression of the fDHFR-UbR48-Glu455-Rec8f fusion, which is processed in the extract to yield fDHFR-UbR48 and the Glu455-Rec8f protein, the main product of the cleavage of full-length Rec8 by separase. Note the presence of a prominent protein band, inferred to be the 16-kDa FLAG-tagged Glu455-Rec8f protein, in the presence of the Arg-Ala dipeptide (lane 9) but neither in the absence of added dipeptide nor in the presence of Ala-Arg (lanes 8 and 10; see also “Experimental Procedures”). Also indicated in D and E, are the molecular masses of key protein species, the C-terminally FLAG-tagged Rec8 fragment (16 kDa) and the N-terminally FLAG-tagged reference protein DHFR-Ub (33 kDa). An asterisk denotes a protein band that cross-reacted with anti-FLAG antibody.
