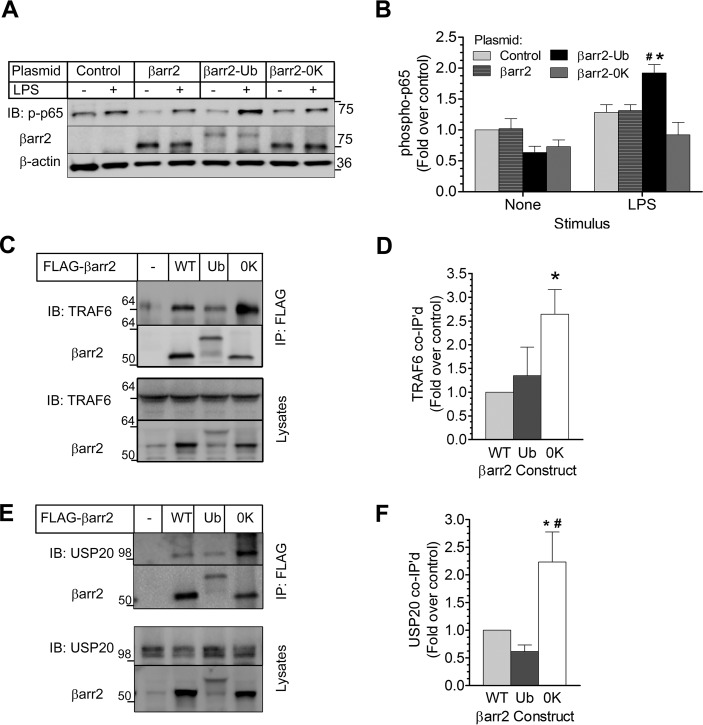
FIGURE 11.
Reciprocal roles of βarr2 in NFκB signaling are defined by the scaffolding efficiency of βarr2 for USP20. A, βarr1−/−/βarr2−/− MEFs were transfected with plasmids encoding YFP (control) or the indicated YFP-tagged construct: WT-βarr2; a βarr2-ubiquitin chimera that is resistant to deubiquitination (βarr2-Ub); or βarr2 in which all Lys residues are mutated to Arg (βarr2–0K). MEFs were stimulated ± LPS (1 μg/ml) for 10 min (37 °C), and extracts were immunoblotted (IB) serially for phospho-p65(Ser-536), βarr2, and β-actin. B, band intensities for p-p65(Ser-536) were normalized to corresponding β-actin band intensities. These ratios were normalized to those obtained from unstimulated, control-transfected βarr1−/−/βarr2−/− MEFs to obtain fold over control, plotted as mean ± S.E. from five independent experiments. Compared with the cognate basal signal (*) or compared with all LPS-stimulated groups (#): p < 0.05. C, HEK-293 cells were transiently transfected with plasmids encoding no protein (−) or the indicated FLAG-tagged construct: βarr2 WT, βarr2-Ub (Ub), or βarr2–0K (0K). βarr2 immunoprecipitates and cognate lysates were immunoblotted serially for (endogenous) TRAF6 and βarr2. D, band intensities for co-immunoprecipitated TRAF6 were normalized to corresponding βarr2 band intensities. These ratios were normalized to those obtained in WT βarr2 IPs to obtain fold over control, plotted as mean ± S.E. from three independent experiments performed in triplicate. *, p < 0.05 compared with βarr2 WT or with βarr2-Ub. E, HEK-293 cells were transfected and immunoprecipitated as in C, but βarr2 immunoprecipitates and cognate whole cell lysates were immunoblotted serially for (endogenous) USP20 and βarr2. F, band intensities for co-immunoprecipitated USP20 were normalized to corresponding βarr2 band intensities. These ratios were normalized to those obtained in WT βarr2 IPs to obtain fold over control, plotted as mean ± S.E. from three independent experiments performed in triplicate. *, p < 0.05 compared with βarr2 WT; #, p < 0.01 compared with βarr2-Ub.