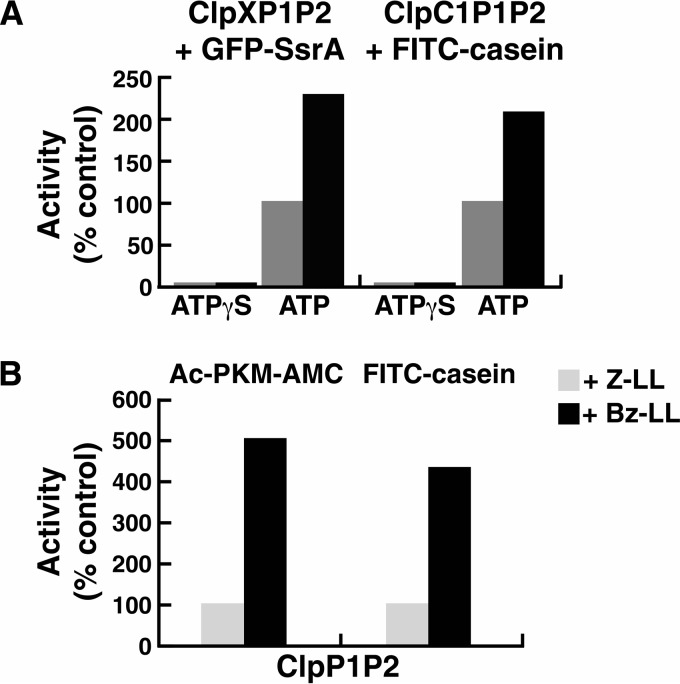
FIGURE 5.
Bz-LL stimulates proteolytic activity of ClpP1P2 more than Z-LL in the presence or absence of the regulatory ATPases. A, Bz-LL enhanced the ability of ClpC1 or ClpX to promote protein degradation by ClpP1P2. Degradation of FITC-casein by ClpP1P2 in the presence of ClpC1 and degradation of GFP-SsrA by ClpP1P2 in the presence of ClpX were measured continuously as described under “Experimental Procedures” in buffer A containing either 2 mm Mg-ATP or 100 μm ATPγS and the dipeptide activators as indicated. For ease of comparison, data were normalized to the rate of degradation in the presence of Z-LL, which was taken as 100% (control). Each point is the average of 3 measurements, which agreed within 5–10%. Similar results were obtained in at least three independent experiments. B, Bz-LL promotes degradation of peptides and unfolded proteins by ClpP1P2 in the absence of ATPases. FITC-casein was incubated with a mixture of ClpP1 and ClpP2 in the presence of 2 mm Bz-LL or 5 mm of Z-LL. Degradation was monitored by the decrease in fluorescence of FITC-casein as described under “Experimental Procedures.” Peptidase activity was measured as described in Fig. 1. Protein degradation or peptidase activity in the presence of Z-LL was designated as 100% (control). Each point is the average of 3 measurements, which agreed within 5–10%. Similar results were obtained in at least three independent experiments.