FIGURE 6.
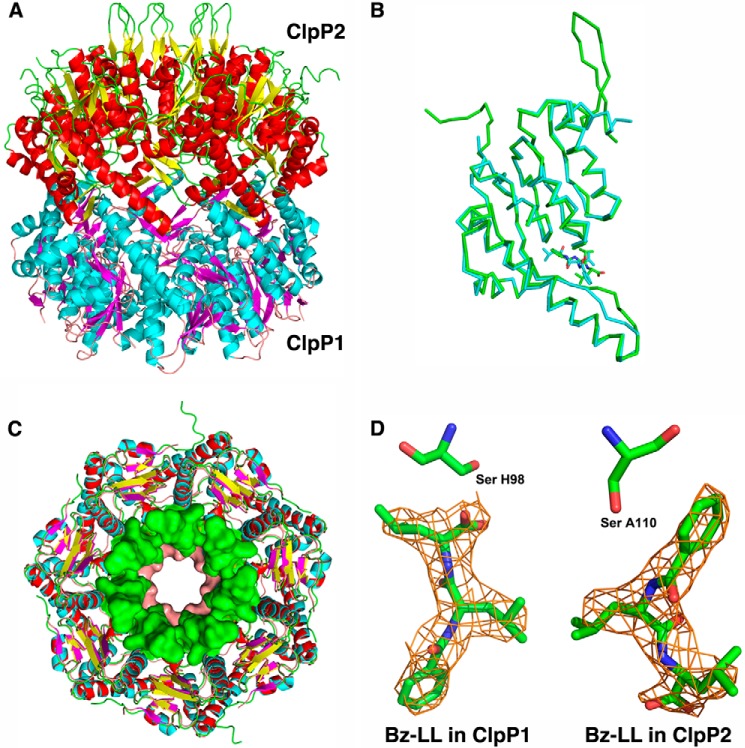
Structural features of Mtb ClpP1P2. A, the tetradecamer is a complex of ClpP1 and ClpP2 heptamers. The mixed tetradecamer formed by joining the heptamers of ClpP1 (cyan helices and magenta β-strands) and ClpP2 (red helices and yellow β-strands) is shown in side view in schematic representation. In ClpP2 the N-terminal β-hairpins are in an extended conformation (yellow and green). B, ribbon representation showing the overlap of ClpP1 and ClpP2 subunits. Backbone atoms between residues 20–185 align with root mean square deviation of 1.2 Å. ClpP2 (green ribbon) has an extended N-terminal β-hairpin that is shorter and disordered in ClpP1 (cyan). ClpP2 also has a bulge in the coiled portion of the alpha-β handle that forms the interface between the heptamers in the tetradecameric complex. The activators are depicted as narrow sticks colored according to the subunits to which they are bound. C, overlap of the ClpP1 and ClpP2 heptamers. Stick representations of ClpP1 and ClpP2 are overlaid. Residues 13–19 of ClpP1 and 15–32 of ClpP2 are shown in surface rendering to illustrate the difference in the axial channel diameter in the two heptamers. D, 2Fo − Fc electron density of the activator bound in ClpP1 and ClpP2. The molecules lie in opposite orientations relative to the catalytic serine shown as sticks.