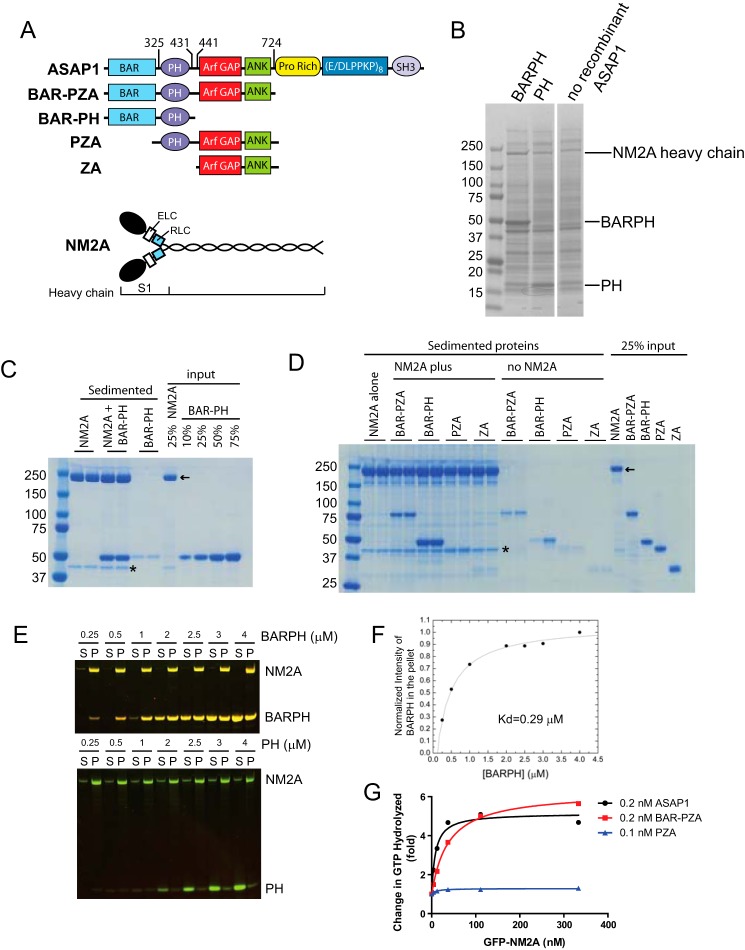
FIGURE 1.
ASAP1 directly interacts with NM2A. A, schematic representations of ASAP1 and NM2A domain structure and recombinant proteins used in this study. Targeting of ASAP1 to membranes is mediated primarily by the Pro-rich and SH3 domains. The PH domain regulates GAP activity of ASAP1. B, Coomassie Blue-stained SDS-PAGE gel of proteins sedimented with sucrose-loaded LUVs alone (no recombinant ASAP1) or coated with BAR-PH or PH. The image is a gel in which two central lanes were removed. The image of the gel is otherwise unaltered. C, co-sedimentation of ASAP1 and purified platelet NM2A. BAR-PH and NM2A were incubated either alone or together as indicated. Reaction mixtures were centrifuged and sedimented proteins were separated by SDS-PAGE and visualized with Coomassie Blue stain. Included in the gel were 25% of the NM2A in the reaction and the indicated percent of BAR-PH in the reaction mixture. Reactions were run in duplicate, and one experiment of three is shown. D, co-sedimentation of ASAP1 fragments (1 μm) with NM2A. The indicated fragments of ASAP1 were incubated with or without 0.6 μm NM2A and sedimented by centrifugation. Reactions were run in duplicate with the indicated proteins. The sedimented proteins and 25% input were separated by SDS-PAGE and visualized with Coomassie Blue stain. Asterisk indicates actin. Arrow indicates NM2A. Different fragments of ASAP1 were annotated in the schematic of ASAP1 domain structure. E and F, concentration-dependent ASAP1-NM2A association. NM2A was expressed in and purified from Sf9 cells. BAR-PH was titrated into a binding reaction containing 0.6 μm NM2A. At high concentrations of BAR-PH, some BAR-PH remains in the supernatant (S), indicating saturated binding. P, pellet. PH remains in the supernatant at all concentrations tested. The data of BAR-PH, after background corrections, were fit to an equation for one site binding. G, purified NM2A stimulates ASAP1 GAP activity dependent on BAR domain. GFP-NM2A purified from Sf9 cells was titrated into Arf GAP reactions containing the indicated recombinant ASAP1.