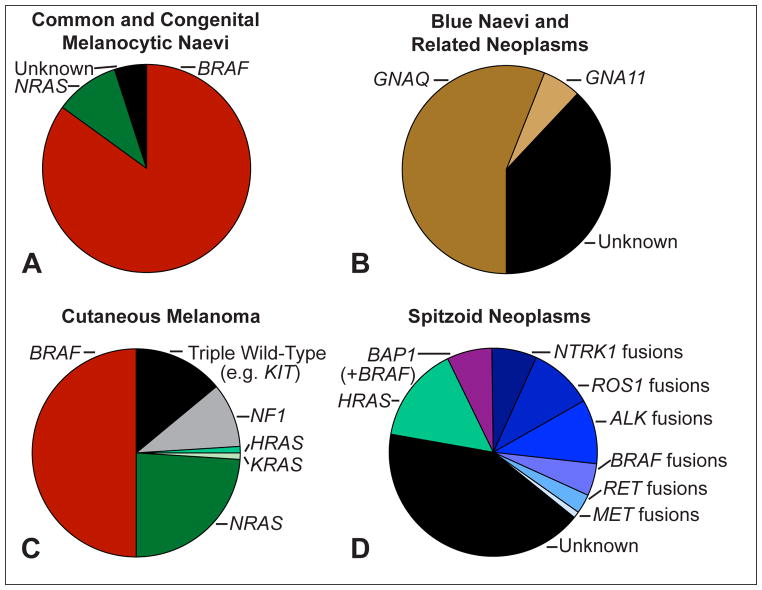
Fig. 1.
Frequent genomic aberrations in cutaneous melanocytic tumours. (A) Common acquired naevi show BRAF hotspot mutations in the vast majority of cases. Congenital naevi frequently harbour NRAS hotspot mutations. (B) Blue naevi and related melanocytic neoplasms share activating mutations of GNAQ and GNA11. While GNAQ mutations dominate in cutaneous proliferations, GNA11 and GNAQ mutations account each for ~40% of uveal melanomas. (C) Cutaneous melanomas show BRAF hotspot mutations in ~50%, NRAS mutations in ~25% and rarely KRAS and HRAS mutations, and NF1 loss in ~10% of cases. The ‘triple wild-type melanoma’ subtype is a heterogeneous subgroup of melanoma with infrequent driver mutations such as KIT, CTNNB1, or genomic rearrangements. (D) Compared to cutaneous melanoma and to common and blue naevi, Spitz tumours show different chromosomal aberrations: translocations involving the kinases ALK, ROS1, NTRK1, BRAF, RET, MET are observed in up to 50% of cases, but are rarely observed in other subtypes of melanocytic tumours. HRAS mutations are often associated with a desmoplastic histological phenotype (desmoplastic Spitz naevus). Aberrations of BAP1 occur frequently with activating BRAF mutations and are associated with an epithelioid morphology.