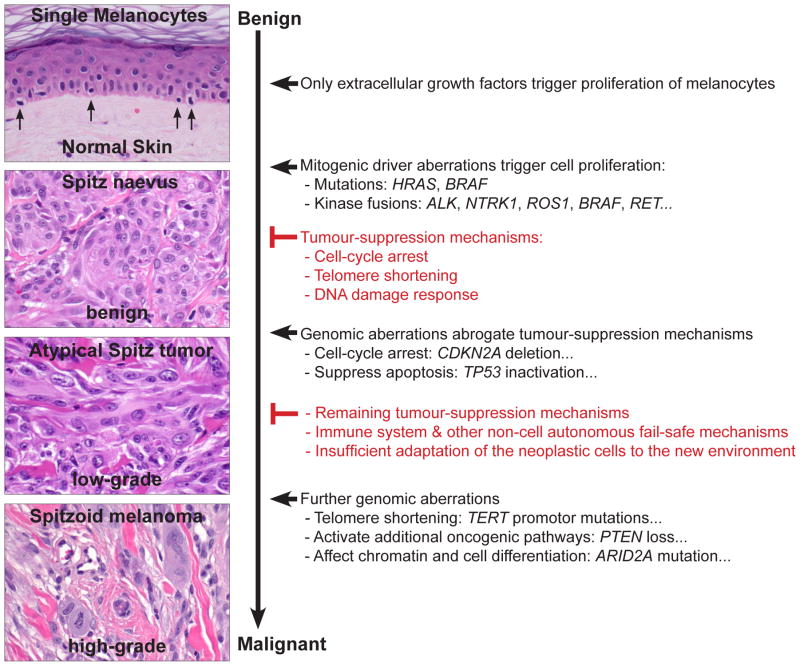
Fig. 9.
Tumour progression model of Spitz tumours. Most tumours, including melanocytic neoplasms, develop through sequential acquisition of genomic aberrations. This tumour progression model suggests a continuous biological tumour spectrum rather than a clear dividing line between benign and malignant. The acquisition of genomic aberrations usually correlates with increased histological atypia and, consequently, tumours with conflicting histological criteria (atypical Spitz tumours) show more genomic aberrations than benign (Spitz naevi), but fewer than malignant (spitzoid melanoma) tumours. The order of genetic aberrations described here is likely to be a common sequence, because cell proliferation (induced by mitogenic genetic aberrations) is associated with a high probability to acquire additional genetic aberrations. However, the genetic changes may also occur in a different order and the sequence displayed here denotes that spitzoid melanomas have additional mutations, but not that all melanomas arise from naevi. Spitzoid melanoma arising without an obvious antecedent naevus may suggest that aberrations in the fail-safe mechanisms develop before the mitogenic driver. Spitz naevi usually only have a strong proliferation signal such as activating HRAS mutations or kinase fusions. These mitogenic genetic aberrations initiate tumour formation, but after initial cell proliferation, multiple fail-safe mechanisms stably block further growth (Spitz naevus). Atypical Spitz tumours abrogate some of these fail-safe mechanisms by gaining additional genomic aberrations so that the cells may continue to grow or to survive in distant organs, such as the lymph nodes. For example, aberrations of CDKN2A, CDK4, or CCND1 undermine the cell-cycle arrest, and TERT promoter mutations may prevent telomere shortening, and consequently senescence. The acquisition of these aberrations is reflected by an increase of cytological and histological atypia. Spitzoid melanomas acquire even more genetic and epigenetic aberrations, which may activate additional oncogenic pathways, affect the chromatin landscape, or reduce cell differentiation, so that the neoplastic cells may colonise and replace the infiltrated organs.