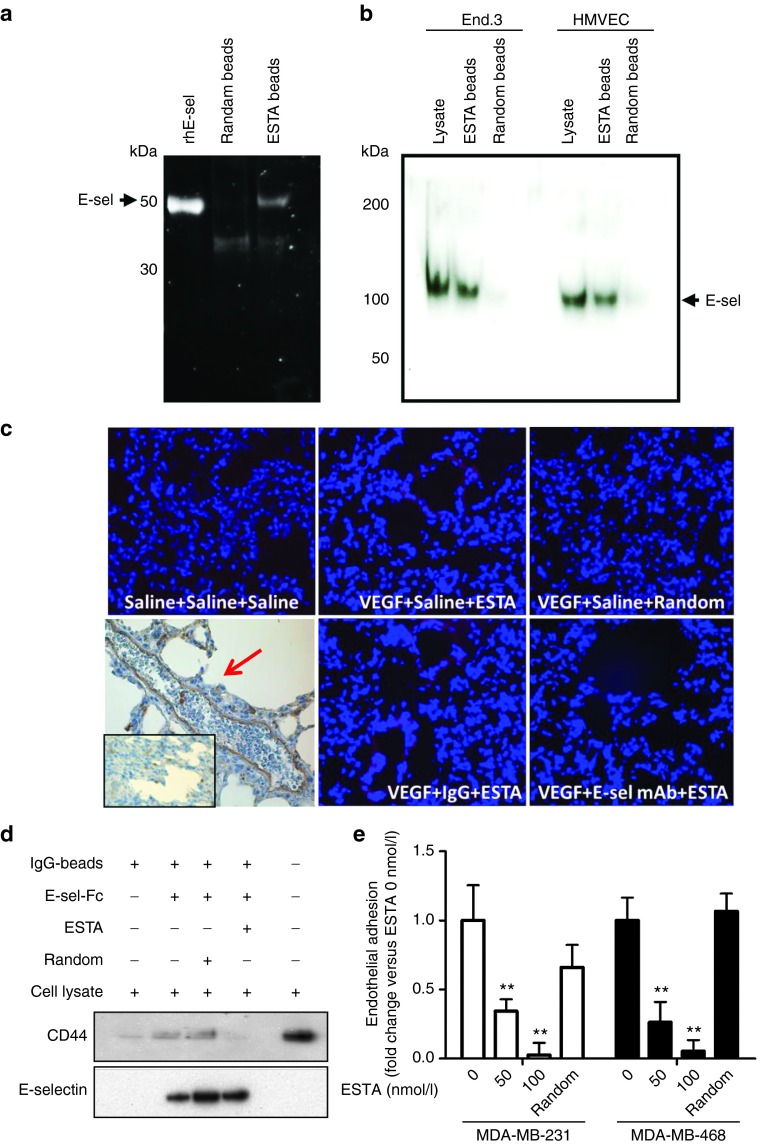
Figure 3.
ESTA is a functional antagonistic ligand of E-selectin. (a) Physical interaction of ESTA with recombinant human E-selectin. ESTA or random aptamer conjugated silica beads were incubated with the external domain of rhE-selectin. The ESTA-conjugated silica beads were incubated with SDS sample buffers to release the protein, separated by SDS–PAGE, and visualized by SYPRO RUBY. Arrows indicate the rhE-selectin. (b) Physical interaction of ESTA with human and mouse plasma membrane E-selectin. Plasma membrane proteins were isolated from human (HMVEC) and mouse (End.3) endothelial cell lines to test the binding to the ESTA or random aptamer conjugated silica beads. Arrows indicate the E-selectin (110 kDa) extracted from plasma membranes. (c) The binding of ESTA to E-selectin expressing lung capillaries. Six-week-old athymic nu/nu mice were preconditioned with i.v. injection of VEGF (100 ng). E-selectin expression in the lung capillaries was confirmed by immunohistochemistry as indicated by the red arrow. Three hours after VEGF or saline injection, the mice were intravenously injected with either saline, rat antimouse E-selectin monoclonal antibody, or rat IgG (100 µg). Three hours later, the mice were intravenously injected with Cy3-labeled ESTA (100 µg). Tissues were harvested 12 hours later and 6-µm frozen sections of lung tissue were counterstained with Hoechst 33342. (d) ESTA inhibits physical interaction of CD44 and E-selectin. IgG magnetic beads coupled with rhE-selectin-FC chimera were preincubated with ESTA (50 nmol/l) for 30 minutes and then incubated with MDA-MB-231 cell lysate (500 µg) at 4 °C for 1 hour. After a brief wash with lysis buffer, protein was eluted from the beads and subjected to western blot. (e) ESTA inhibits the shear-resistant adhesion of CD44+ breast cancer cells to E-selectin expressing HMVEC. A monolayer of E-selectin inducible Tet-on HMVEC grown on a flow chamber was incubated with doxycycline for 5 hours and then pretreated with ESTA (50 or 100 nmol/l) or random aptamer (100 nmol/l) for 30 minutes at 37 °C. The adhesion of CD44+ breast cancer cells (MDA-MB-231 and MDA-MB-468 at 105cells/ml) was infused into a chamber at 1 dyn/cm2 for 5 min and the cancer cell adhesion to HMVEC was counted and compared with that of untreated HMVEC (0 nmol/l). The data represent mean ± SD. Statistical significance was determined by nonparametric Student's t-test.