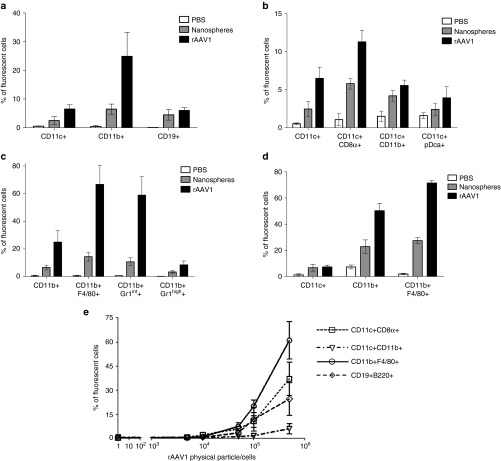
Figure 3.
Antigen-presenting cells (APCs) targeting by rAAV1. (a–d) C57BL/6 mice were injected IV in the retro-orbital sinus with 1013 pp of nanospheres (gray bars) or same amount of Alexa Fluor-labeled rAAV1 (black bars) or with equivalent volume (100 µl) of phosphate-buffered saline (PBS) (open bars). Two hours later, various populations of cells were sorted with magnetic beads from the spleen (CD11c+, CD11b+, CD19+ cells) (as indicated in Supplementary Figure S2a) or from the liver (CD45+ cells) of mice to measure the presence of fluorescent particles by FACS. Prior to FACS analysis, sorted cells were stained with noninterfering fluorescence to recognize the CD8α, CD11b, pDC, F4/80, and Gr1 subsets. Data are expressed as the mean percentage of fluorescent cells ± SD obtained in three independent experiments, each including two or three mice per group, testing two different batches of rAAV1. Measures of particle targeting were made: (a) in total spleen CD11c+ CD11b+ and CD19+ cells (b) in subsets of the sorted CD11c+ spleen cells as identified by multi-color staining as indicated (c) in subsets of the sorted CD11b+ spleen cells as identified by multi-color staining (d) in subsets of the sorted liver CD45+ cells identified by multi-color staining. (e) In vitro rAAV1 binding assay. Splenic CD11c+, CD11b+, and CD19+ cells were obtained from untreated C57BL/6 mice using the same purification technique as above and cells were incubated 1 hour at 4 °C with different amounts of Alexa-fluor 488-stained rAAV1 vector and the percentage of fluorescent cells was measured by FACS. Data are representative of one experiment out of five. FACS, fluorescence activated cell sorting.