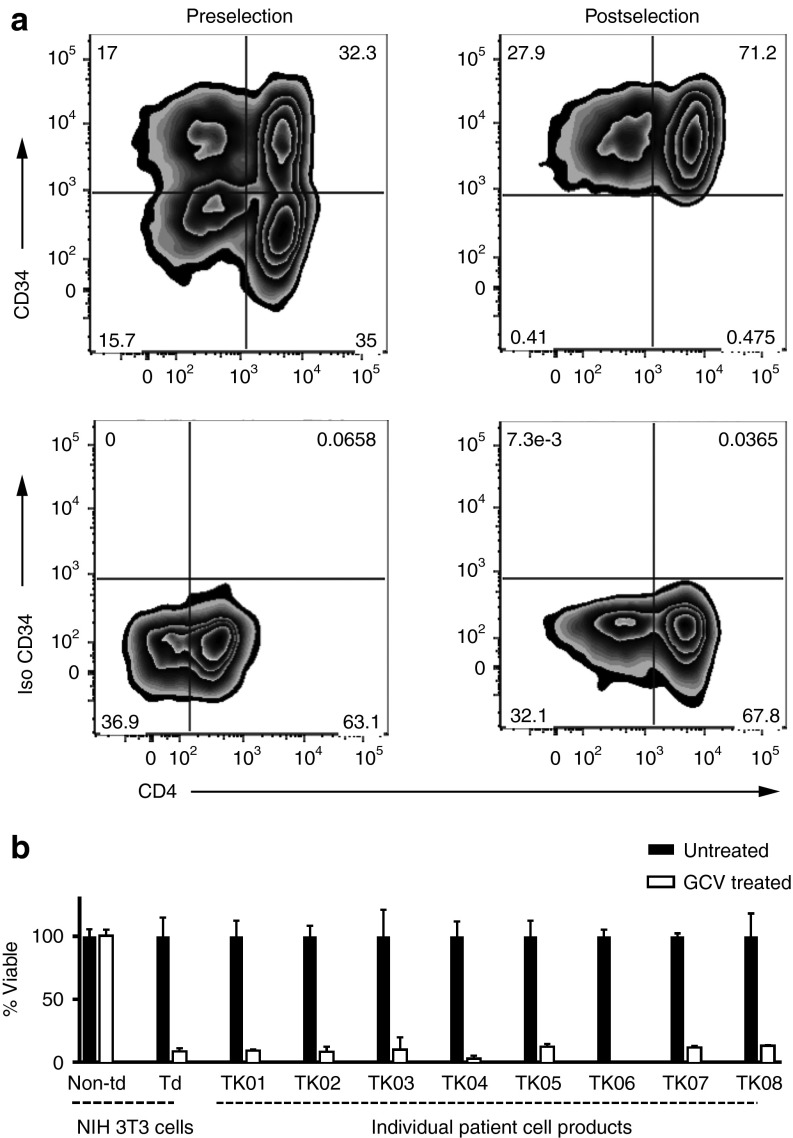
Figure 2.
Product purification and validation. (a) Flow cytometry was used to examine CD3+ cells for CD34 and CD4 expression to determine purity, transduction efficiency (% CD3+CD34+ cells/total CD3+ cells), and subtypes in all patient products (representative cell product for patient TK07). Upper left: CD3+CD34+ T cells prior to selection on the CliniMacs system. Upper right: CD34+ T cells postselection. Lower panels: isotype controls. (b) Survival of cells after incubation in 1 µmol/l ganciclovir for 5–7 days. The control used NIH 3T3 cells (nontransduced (Non-td) and ΔU3CD34-TK75-transduced (Td)). The donor T cells in individual patient cell products were activated for 48 hours in the presence of 1 anti-CD3/anti-CD28-coated bead per cell. After 5–7 days viability was assessed. Viability relative to untreated cells is shown. Data for control NIH 3T3 cells is pooled from all experiments. Error bars represent standard deviation.