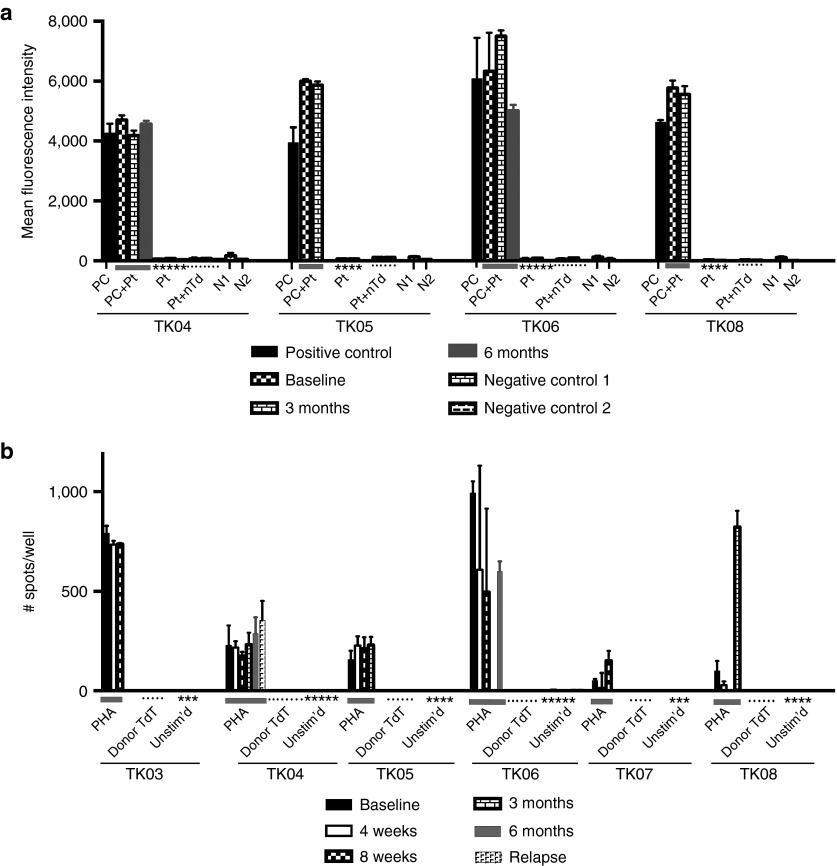
Figure 5.
Assessment of patient immune response against TdT. (a) Reactivity of antibodies in patient serum with nontransduced and ΔU3CD34-TK75-transduced murine NIH 3T3 cells. Patient samples were collected at baseline, 3, and 6 months. Patients TK01, TK02, TK03, and TK07 were not surviving at 3 months. Only patients TK04 and TK06 were alive at 6 months. Transduced NIH 3T3 cells were exposed to murine anti-huCD34 antibody (positive control (PC)) or to patient serum (Pt) followed by a PE-tagged anti-human or murine IgG before analysis by flow cytometry. PC+Pt = the positive control supplemented with patient serum from each time point. N1 = Nonspecific binding of the secondary PE-anti-murine IgG to the cells in the absence of primary antibody. N2 = the same using PE-anti-human IgG. Pt+nTd= Patient's serum incubated with nontransduced NIH 3T3 cells followed by secondary PE-anti-human IgG. (b) Elispot assay to detect patient cells producing γ-IFN in response to transduced donor cells. Unstimulated patient peripheral blood mononuclear cells (PBMCs) or PBMC stimulated with PHA (1.2 µg/ml) or irradiated TdT from the donor product administered to the patient were tested in an ELISPOT assay for production of γ-IFN at various times. Patients TK01 and TK02 were not surviving at 4 weeks. Insufficient numbers of cells were available to test PBMC from patient TK06 at 3 months or patient TK08 at 8 weeks.