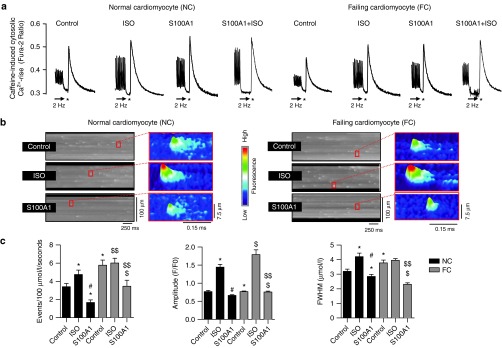
Figure 2.
S100A1 attenuates physiological and abrogates pathological diastolic RyR2 activity. (a) Representative tracings of electrically-stimulated (2 Hz; arrow) steady-state Ca2+ transients from basal and ISO-stimulated (100 nmol/l) control (AdGFP) and S100A1-treated (AdS100A1) NCs (left) and FCs (right). Asterisk (*) indicates the 10 mmol/l caffeine pulse for semiquantitative assessment of the SR Ca2+ content due to the caffeine-mediated maximal rise of the cytosolic Ca2+ signal. Note that both ISO and S100A1 enhance the SR Ca2+ load in NCs and FCs (see Supplementary Figure S1d for statistical analyses). (b) Representative fluorescent line-scan and magnified surface plot images of elementary Ca2+ release events in control (AdGFP-transfected), isoproterenol-stimulated (ISO, 100 nmol/l, AdGFP-transfected) and S100A1 (AdS100A1-transfected) resting normal (NCs, left panel) and failing rat cardiomyocytes (FCs, right panel). (c) Corresponding statistical analysis of Ca2+ spark characteristics (frequency, amplitude, and width) shows that FCs exhibit significantly greater SR Ca2+ leak than NCs. S100A1 attenuates RyR2-mediated Ca2+ leak in NCs and FCs. n = 90 cells in each group derived from four different isolations. *P < 0.05 versus NC control, #P < 0.05 versus NC ISO, $P < 0.05 versus FC control, $$P < 0.05 versus FC ISO. Data are given as mean ± SEM.