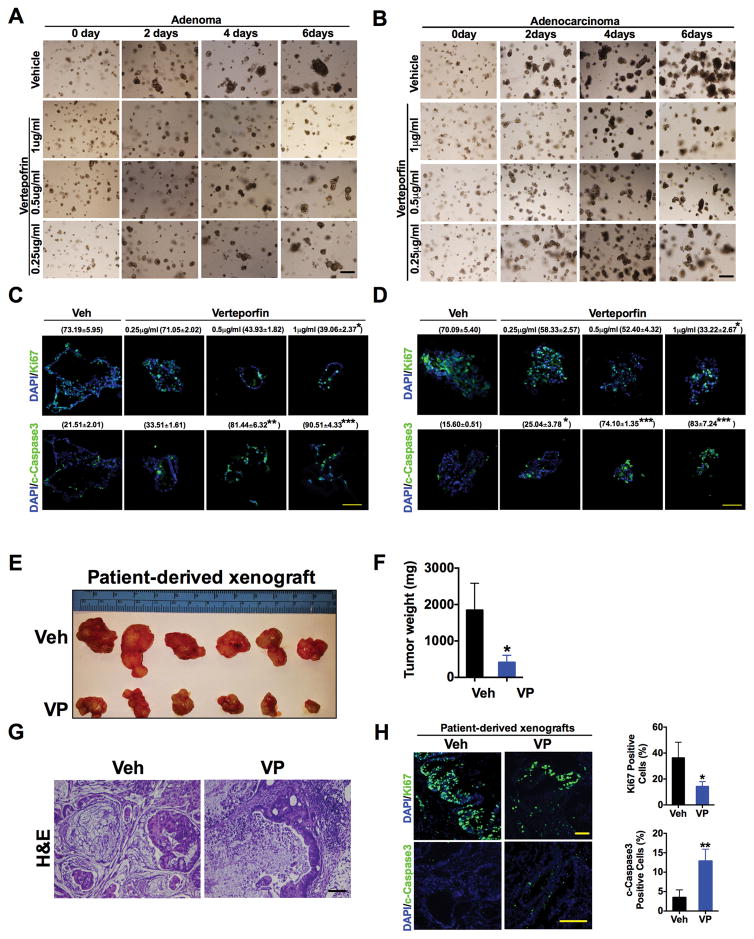
Fig. 3. Verteporfin decreases cell growth in patient-derived enteroid and xenograft models.
(A and B) Representative images of (A) adenoma or (B) adenocarcinoma patient-derived enteroids treated with verteporfin (VP) for the indicated doses and times. (C and D) Immunofluorescence staining and quantification (% positive staining ± S.E.M, n=3 enteroids of Ki67 and cleaved caspase3 in patient-derived (C) adenoma or (D) adenocarcinoma enteroids in the presence of vehicle (Veh) or VP for 6 days. (E to H) Size (E), weight (F), H&E staining (G), and proliferative and apoptotic analysis (H) of CRC patient-derived xenografts in mice treated with vehicle (n=6) or VP (n=8). Data are mean ± S.E.M of at least three independent experiments. *p < 0.05; **p<0.01; ***p<0.001 vs Veh. Scale bars, 50μm.