Abstract
The aim of the present study was to investigate the role of comorbidities in the outcomes of patients with peripheral T-cell lymphoma (PTCL) in a Chinese population. Fifty-six newly diagnosed PTCL patients aged >60 years were enrolled in our institution between April 2008 and August 2014. Medical record details including clinical parameters, pathological status, and treatment were reviewed. Prognostic factors were assessed using univariate and multivariate analyses. Forty-one (73.2%) patients with PTCL, not otherwise specified (PTCL-NOS), nine (16.1%) with angioimmunoblastic T-cell lymphoma, and six (10.7%) with anaplastic large cell lymphoma were recruited in this study. Twenty-eight (50%) had at least one comorbidity. Univariate analysis showed that an Eastern Cooperative Oncology Group score of 2–4, the presence of B symptoms, an International Prognostic Index (IPI) score of 3–5, and a Charlson Comorbidity Index (CCI) score ≥2 were significantly associated with shortened overall survival (OS), whereas the presence of B symptoms, an IPI of 3–5, and a CCI ≥2 were associated with worsened progression-free survival (PFS). Multivariate analysis indicated that a high CCI (≥2) and a high IPI (3–5) were poor independent prognostic factors for OS and PFS in the elderly patients with PTCL. Comorbidity was identified as a new independent poor prognostic factor for elderly patients with PTCL.
Keywords: comorbidity, peripheral T-cell lymphoma, OS, PFS, prognostic factor
Introduction
Peripheral T-cell lymphoma (PTCL) is a heterogeneous group of non-Hodgkin’s lymphomas with the characteristics of rare incidence, aggressive course, and poor survival.1 PTCL, not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), and anaplastic large cell lymphoma (ALCL) are the major PTCL subtypes in Asia, representing ~40% of cases.2 The cyclophosphamide, adriamycin, vincristine, and prednisone (CHOP) regimen is widely used as a first-line treatment for PTCL, but the outcomes are very poor with a 5-year overall survival (OS) rate of ~30%.1
With the increase in life expectancy, increasingly more patients with PTCL are >60 years of age, and those patients always have one or more comorbidities such as diabetes or cerebrovascular disease, which not only influence the prognosis of the disease itself but may also affect the treatment choice.3–5 Therefore, a single numeric score of comorbidity, based on the collection of several comorbidities, was a very useful and important method, which can reduce the number of candidate variables into a manageable set of proxy variables.6,7 The Charlson Comorbidity Index (CCI) is the most widely used comorbidity index.5,8
An analysis to determine whether the CCI can predict the survival of elderly patients with PTCL has not yet been performed. It was reported that the CCI is a useful index to predict outcomes in nonhematologic9–11 and hematologic malignancies.12–14 Therefore, this retrospective study analyzed patients’ clinical parameters and treatment outcomes to determine the impact of CCI on elderly patients with PTCL.
Materials and methods
Patient selection
In this study, 56 patients aged >60 years with newly diagnosed PTCL were recruited between April 2008 and August 2014. The last follow-up date was March 31, 2015. According to the World Health Organization classification,15 the PTCL-NOS, ALCL, and AITL subtypes were included in this study. The study was approved by the Research Ethics Committee of Tianjin Cancer Hospital, People’s Republic of China. Written informed consent was obtained from the patients prior to their participation.
Data collection
The patients’ medical records were reviewed for their clinical, pathological, and treatment data. Each patient’s history; performance status; blood routine; liver function; renal function; lactate dehydrogenase (LDH); computed tomography scans of the thorax, abdomen, and pelvic cavity; bone marrow aspiration and biopsy; and cognitive status were documented. International Prognostic Index (IPI) scores were based on age, Eastern Cooperative Oncology Group (ECOG) performance status, LDH concentrations, number of extranodal sites involved, and Ann Arbor stage. The treatment responses were classified as complete remission (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to the International Working Group criteria.16 In this study, hemoglobin <120 g/L was defined as low, LDH >245 U/L was defined as high, and β-microglobulin (β2-MG) >2.2 mg/L was defined as high. The CCI score of each patient was calculated according to their past and present medical history.8,17
Statistical analysis
A statistical analysis was performed using SPSS 19.0 soft-ware (SPSS Inc., Chicago, IL, USA). OS was calculated from the diagnosis until death from any cause, while progression-free survival (PFS) was calculated from the diagnosis until disease progression or death. The survival curve was calculated by the log-rank test. Multivariate analysis was performed using the Cox proportional hazards regression model. The GraphPad Prism software package was used to create the Kaplan–Meier estimate graphs. Values of P<0.05 were considered statistically significant.
Results
Patient characteristics
A total of 34 males (60.7%) and 22 females (39.3%) with a median age of 67 years (range: 60–80 years) were included in this study. With regard to histological subtypes, 41 (73.2%) patients had PTCL-NOS, nine (16.1%) had AITL, and six (10.7%) had ALCL. All of the patients were divided into the low CCI (score 0 or 1) and high CCI (score ≥2) groups. Thirteen patients were included in the high CCI group. The patients’ clinical parameters and histological features are summarized in Table 1.
Table 1.
Clinical characteristics of patients with PTCL
| Characteristic | No of patients (%) |
|---|---|
| Sex | |
| Male | 34 (60.7) |
| Female | 22 (39.3) |
| Age (years) | |
| ≤70 | 38 (67.9) |
| >70 | 18 (32.1) |
| Performance status | |
| ECOG 0–1 | 32 (57.1) |
| ECOG 2–4 | 24 (42.9) |
| Pathological type | |
| PTCL-NOS | 41 (73.2) |
| AITL | 9 (16.1) |
| ALCL | 6 (10.7) |
| LDH | |
| Normal | 21 (37.5) |
| Elevated | 35 (62.5) |
| Hemoglobin | |
| Normal | 43 (76.8) |
| Low | 13 (23.2) |
| B symptoms | |
| Present | 23 (41.1) |
| Absent | 33 (58.9) |
| β2-MG | |
| Normal | 34 (60.7) |
| Elevated | 22 (39.3) |
| Bone marrow involvement | |
| Yes | 4 (7.0) |
| No | 52 (93.0) |
| Ann Arbor stage | |
| I/II | 17 (30.4) |
| III/IV | 39 (69.6) |
| IPI | |
| 1–2 | 20 (35.7) |
| 3–5 | 36 (64.3) |
| CCI | |
| 0 | 28 (50.0) |
| 1 | 15 (26.8) |
| 2 | 6 (10.7) |
| >2 | 7 (12.5) |
| Treatment | |
| CHOP/CHOP-like regimen | 45 (80.4) |
| Regimen other than CHOP | 11 (19.6) |
| Therapeutic evaluation | |
| CR/PR | 34 (60.7) |
| SD/PD | 22 (39.3) |
Abbreviations: PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified; ALCL, anaplastic large cell lymphoma; AITL, angioimmunoblastic T-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; β2-MG, β-microglobulin; CHOP, combination chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone; IPI, International Prognostic Index; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; CCI, Charlson Comorbidity Index.
Twenty-eight (50%) patients had at least one comorbidity, the most common of which were diabetes and cerebrovascular disease (Table 2). Most patients were treated with CHOP or CHOP-like regimen as the first-line treatment, while six patients were treated with modified cyclophosphamide, doxorubicin, vincristine, and dexamethasone (HyperCVAD) and five were treated with another regimen. Thirty-four patients (60.7%) achieved CR or PR at the end of the treatment, while 22 patients (39.3%) had SD or PD.
Table 2.
Charlson Comorbidity Index of patients with PTCL
| Characteristic | Score | No of patients |
|---|---|---|
| Myocardial infarction | 1 | 2 |
| Congestive heart failure | 1 | 1 |
| Peripheral vascular disease | 1 | 1 |
| Cerebrovascular disease (except | 1 | 7 |
| hemiplegia) | ||
| Dementia | 1 | 1 |
| Chronic pulmonary disease | 1 | 1 |
| Connective tissue disease | 1 | 2 |
| Ulcer disease | 1 | 3 |
| Mild liver disease | 1 | 1 |
| Diabetes without complications | 1 | 12 |
| Diabetes with end-organ damage | 2 | 1 |
| Hemiplegia | 2 | 1 |
| Moderate or severe renal disease | 2 | 0 |
| Second solid tumor (nonmetastatic) | 2 | 0 |
| Leukemia | 2 | 0 |
| Lymphoma* | 2 | 0 |
| Moderate or severe liver disease | 3 | 1 |
| Second metastatic solid tumor | 6 | 0 |
| Acquired immunodeficiency syndrome | 6 | 0 |
Note:
Excluded from analysis.
Abbreviation: PTCL, peripheral T-cell lymphoma.
Prognostic factor analysis
Each patient’s medical records were retrospectively reviewed. The patients were divided into two subgroups according to age (≤70 vs >70 years), sex (male vs female), ECOG score (0–1 vs 2–4), hemoglobin (normal vs low), B symptoms (present vs absent), Ann Arbor stage (I–II vs III–IV), β2-MG (normal vs elevated), bone marrow involvement (positive vs negative), LDH concentrations (normal vs elevated), IPI (1–2 vs 3–5), and CCI (0–1 vs ≤2).
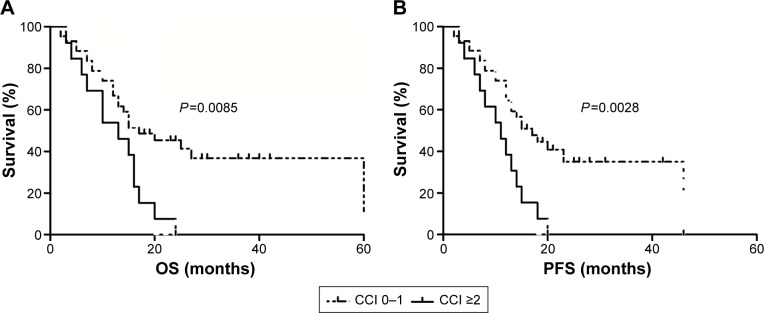
Univariate analysis showed that an ECOG score of 2–4, the presence of B symptoms, an IPI of 3–5, and a CCI ≥2 were significantly associated with worse OS, while the presence of B symptoms, an IPI of 3–5, and a CCI ≥2 were associated with shortened PFS. Multivariate analysis indicated that a high CCI and a high IPI were poor independent prognostic factors for patients with PTCL (Tables 3 and 4). OS and PFS in the high CCI group were significantly shorter than those in the low CCI group (P=0.0085 and P=0.0028, respectively; Figure 1).
Table 3.
Univariate and multivariate analyses of the prognostic risk factors for overall survival in PTCL
| Variable | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| ECOG (0–1 vs 2–4) | 1.84 (0.25–3.56) | 0.031 | 1.38 (0.82–3.15) | 0.635 |
| Ann Arbor stage (I–II vs III–IV) | 1.24 (1.35–4.67) | 0.134 | ||
| B symptoms (absent vs present) | 2.57 (0.45–7.89) | 0.032 | 0.82 (0.34–2.18) | 0.514 |
| β2-MG (normal vs elevated) | 0.73 (0.46–3.24) | 0.572 | ||
| LDH (normal vs elevated) | 0.59 (0.14–2.31) | 0.786 | ||
| IPI (1–2 vs 3–5) | 4.12 (1.59–6.45) | 0.028 | 4.12 (1.36–6.72) | 0.01 |
| CCI (0–1 vs ≥2) | 3.24 (0.73–6.45) | 0.019 | 3.56 (0.82–4.51) | 0.006 |
| Therapeutic evaluation (CR/PR vs SD/PD) | 2.38 (0.62–6.42) | 0.673 | ||
Abbreviations: PTCL, peripheral T-cell lymphoma; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; β2-MG, β-microglobulin; LDH, lactate dehydrogenase; IPI, International Prognostic Index; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; CCI, Charlson Comorbidity Index Score.
Table 4.
Univariate and multivariate analyses of the prognostic risk factors for progression-free survival in PTCL
| Variable | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| ECOG (0–1 vs 2–4) | 1.32 (1.02–4.32) | 0.071 | ||
| Ann Arbor stage (I–II vs III–IV) | 1.48 (0.58–3.64) | 0.233 | ||
| B symptoms (absent vs present) | 2.72 (1.63–6.42) | 0.045 | 1.46 (0.68–2.89) | 0.472 |
| β2-MG (normal vs elevated) | 1.29 (0.71–3.87) | 0.592 | ||
| LDH (normal vs elevated) | 0.78 (0.43–3.20) | 0.134 | ||
| IPI (1–2 vs 3–5) | 2.94 (1.52–5.61) | 0.032 | 3.01 (0.98–5.61) | 0.017 |
| CCI (0–1 vs ≥2) | 2.39 (0.54–3.72) | 0.016 | 2.99 (1.14–4.58) | 0.008 |
| Therapeutic evaluation (CR/PR vs SD/PD) | 2.69 (0.35–6.27) | 0.187 | ||
Abbreviations: PTCL, peripheral T-cell lymphoma; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; β2-MG, β-microglobulin; LDH, lactate dehydrogenase; IPI, International Prognostic Index; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; CCI, Charlson Comorbidity Index Score.
Figure 1.
Ability of the Charlson Comorbidity Index (CCI) score to predict overall survival (OS) and progression-free survival (PFS) of elderly patients with peripheral T-cell lymphoma.
Notes: Both OS (A) and PFS (B) in the high CCI group were significantly shorter than those in the low CCI group (P=0.0085 and P=0.0028, respectively).
Discussion
PTCL encompasses a subset of rare and usually aggressive T-cell diseases that account for ~12% of cases of non-Hodgkin lymphomas in Western countries.18 However, in Asia, the incidence was higher, >20%.19,20 Due to its rarity and lack of standardized treatment strategies, its prognosis remains very poor.
The CCI is the most commonly applied comorbidity score system, and a high CCI represents shortened survival.11,21 It had been shown that the comorbidity can be regarded as an independent risk factor in several solid and hematological malignancies.10,22,23 Studies on the impact of comorbidity on survival in B-cell non-Hodgkin lymphoma revealed that a high CCI was associated with poor OS.13,24,25
Until now, there have been no data on the ability of the CCI to predict survival in elderly patients with PTCL in a Chinese population. The purpose of this study was to assess comorbidity to evaluate survival among patients with PTCL treated with CHOP or CHOP-like regimen. Univariate analysis showed that the presence of B symptoms, an IPI of 3–5, and a CCI ≥2 were significantly associated with shortened OS and PFS. Multivariate analysis findings suggested that a high CCI was an independent poor risk factor for elderly patients with PTCL.
Conclusion
Comorbidity was considered a poor independent prognostic factor for shortened OS and PFS in patients with PTCL treated with CHOP or CHOP-like regimen. Further studies with larger samples are warranted to confirm our findings.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30672208 and 81270603) and the Tianjin Natural Science Foundation of China (13JCYBJC22800).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Foss FM, Zinzani PL, Vose JM, Gascoyne RD, Rosen ST, Tobinai K. Peripheral T-cell lymphoma. Blood. 2011;117(25):6756–6767. doi: 10.1182/blood-2010-05-231548. [DOI] [PubMed] [Google Scholar]
- 2.Vose J, Armitage J, Weisenburger D, International TCLP. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 3.Hall SF. A user’s guide to selecting a comorbidity index for clinical research. J Clin Epidemiol. 2006;59(8):849–855. doi: 10.1016/j.jclinepi.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Yancik R, Ershler W, Satariano W, Hazzard W, Cohen HJ, Ferrucci L. Report of the National Institute on Aging Task Force on Comorbidity. J Gerontol A Biol Sci Med Sci. 2007;62(3):275–280. doi: 10.1093/gerona/62.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity: a critical review of available methods. J Clin Epidemiol. 2003;56(3):221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 6.Lash TL, Mor V, Wieland D, Ferrucci L, Satariano W, Silliman RA. Methodology, design, and analytic techniques to address measurement of comorbid disease. J Gerontol A Biol Sci Med Sci. 2007;62(3):281–285. doi: 10.1093/gerona/62.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneeweiss S, Maclure M. Use of comorbidity scores for control of confounding in studies using administrative databases. Int J Epidemiol. 2000;29(5):891–898. doi: 10.1093/ije/29.5.891. [DOI] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Singh B, Bhaya M, Stern J, et al. Validation of the Charlson comorbidity index in patients with head and neck cancer: a multi-institutional study. Laryngoscope. 1997;107(11 Pt 1):1469–1475. doi: 10.1097/00005537-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Ouellette JR, Small DG, Termuhlen PM. Evaluation of Charlson-Age Comorbidity Index as predictor of morbidity and mortality in patients with colorectal carcinoma. J Gastrointest Surg. 2004;8(8):1061–1067. doi: 10.1016/j.gassur.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 11.Guzzo TJ, Dluzniewski P, Orosco R, Platz EA, Partin AW, Han M. Prediction of mortality after radical prostatectomy by Charlson comorbidity index. Urology. 2010;76(3):553–557. doi: 10.1016/j.urology.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin TL, Kuo MC, Shih LY, et al. The impact of age, Charlson comorbidity index, and performance status on treatment of elderly patients with diffuse large B cell lymphoma. Ann Hematol. 2012;91(9):1383–1391. doi: 10.1007/s00277-012-1463-9. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi Y, Miura K, Hojo A, et al. Charlson Comorbidity Index is an independent prognostic factor among elderly patients with diffuse large B-cell lymphoma. J Cancer Res Clin Oncol. 2011;137(7):1079–1084. doi: 10.1007/s00432-010-0973-x. [DOI] [PubMed] [Google Scholar]
- 14.Thieblemont C, Grossoeuvre A, Houot R, et al. Non-Hodgkin’s lymphoma in very elderly patients over 80 years. A descriptive analysis of clinical presentation and outcome. Ann Oncol. 2008;19(4):774–779. doi: 10.1093/annonc/mdm563. [DOI] [PubMed] [Google Scholar]
- 15.Tomonaga M. Outline and direction of revised WHO classification of Tumors of Haematopoietic and Lymphoid Tissues. Rinsho Ketsueki. 2009;50(10):1401–1406. Japanese. [PubMed] [Google Scholar]
- 16.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 17.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 18.O’Leary H, Savage KJ. The spectrum of peripheral T-cell lymphomas. Curr Opin Hematol. 2009;16(4):292–298. doi: 10.1097/MOH.0b013e32832b89a9. [DOI] [PubMed] [Google Scholar]
- 19.Kim K, Kim WS, Jung CW, et al. Clinical features of peripheral T-cell lymphomas in 78 patients diagnosed according to the Revised European-American Lymphoma (REAL) classification. Eur J Cancer. 2002;38(1):75–81. doi: 10.1016/s0959-8049(01)00344-6. [DOI] [PubMed] [Google Scholar]
- 20.Ko OB, Lee DH, Kim SW, et al. Clinicopathologic characteristics of T-cell non-Hodgkin’s lymphoma: a single institution experience. Korean J Intern Med. 2009;24(2):128–134. doi: 10.3904/kjim.2009.24.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birim O, Kappetein AP, Goorden T, van Klaveren RJ, Bogers AJ. Proper treatment selection may improve survival in patients with clinical early-stage nonsmall cell lung cancer. Ann Thorac Surg. 2005;80(3):1021–1026. doi: 10.1016/j.athoracsur.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 22.Janssen-Heijnen ML, Houterman S, Lemmens VE, Louwman MW, Maas HA, Coebergh JW. Prognostic impact of increasing age and comorbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol. 2005;55(3):231–240. doi: 10.1016/j.critrevonc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Read WL, Tierney RM, Page NC, et al. Differential prognostic impact of comorbidity. J Clin Oncol. 2004;22(15):3099–3103. doi: 10.1200/JCO.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 24.Janssen-Heijnen ML, van Spronsen DJ, Lemmens VE, Houterman S, Verheij KD, Coebergh JW. A population-based study of severity of comorbidity among patients with non-Hodgkin’s lymphoma: prognostic impact independent of International Prognostic Index. Br J Haematol. 2005;129(5):597–606. doi: 10.1111/j.1365-2141.2005.05508.x. [DOI] [PubMed] [Google Scholar]
- 25.Wildes TM, Augustin KM, Sempek D, et al. Comorbidities, not age, impact outcomes in autologous stem cell transplant for relapsed non-Hodgkin lymphoma. Biol Blood Marrow Transpl. 2008;14(7):840–846. doi: 10.1016/j.bbmt.2008.05.002. [DOI] [PubMed] [Google Scholar]