Fig. 2.
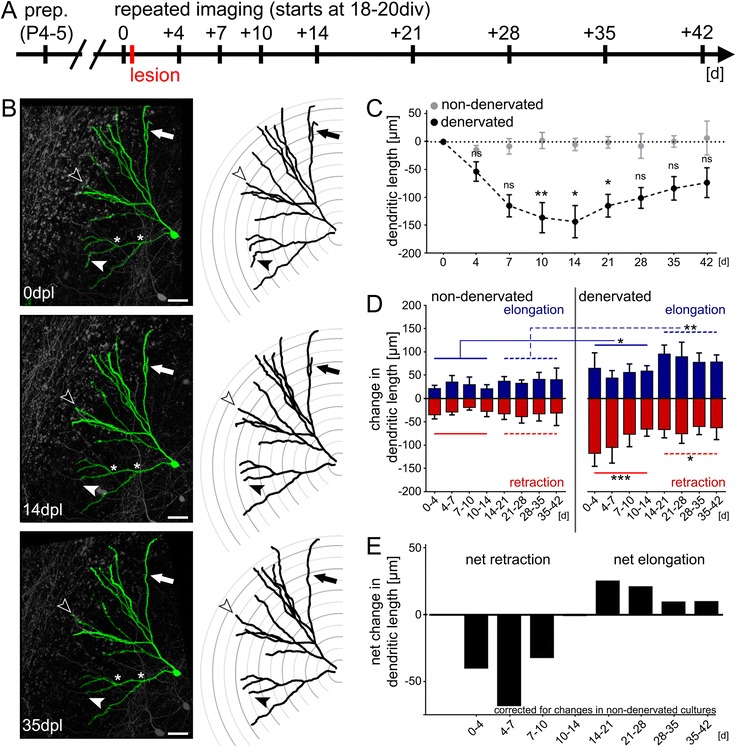
Denervation-induced dendritic remodeling. a Schematic of the experimental design. Slice cultures were prepared at postnatal day 4–5 and allowed to mature for 18–21 days in vitro (div). Repetitive imaging was performed at indicated points in time. Cultures were lesioned immediately after the first imaging session. b Example of a denervated dentate granule cell (2D-projected confocal image stack) and the corresponding reconstructions are shown for 0, 14 and 35 days post lesion (dpl). Note retraction (black arrowhead) and elongation (white arrowhead) of distal dendritic segments. The complete loss of a distal dendritic branch is indicated by the black arrow. Scale bar: 40 μm. c Entorhinal denervation in vitro leads to a reduction in the total dendritic length (TDL) of granule cells, which is followed by a gradual recovery in TDL. No significant change in mean TDL was seen in age- and time-matched non-denervated control cultures imaged in the same way (n = 6 non-denervated cultures vs. n = 8 denervated cultures; Kruskal-Wallis-test followed by Dunn’s post-hoc-test; *, p <0.05; **, p <0.01; ns, not significant). d Analysis of dendritic retraction and elongation reveals a higher degree of dendritic remodeling in denervated cultures. Both retraction and elongation are increased in the denervated group throughout the observation period (Wilcoxon-Mann–Whitney test with pooled data 0-14d and 14-42d; *, p <0.05; **, p <0.01; ***, p <0.001). e Net effects of dynamic changes in the dendritic tree demonstrate net retraction during the early phase and net elongation during the late phase after denervation. Data corrected for changes in non-denervated cultures
