Figure 9. Proline in the M2 helix controls spermine block and permeation .
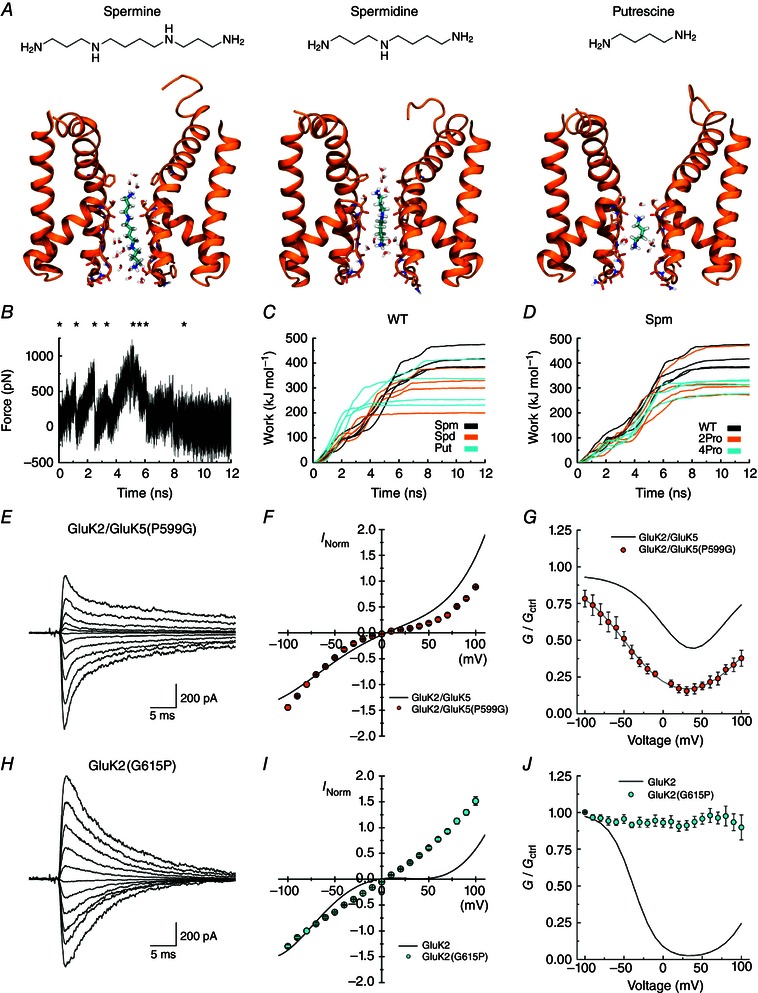
A, each of the three polyamines binding in the NaK filter region, from left to right: spermine (Spm), spermidine (Spd), putrescine (Put). For simplicity, only chains A and C of the protein are included and non‐polar hydrogen atoms of the protein are omitted. Carbon atoms of the ligands are shown in cyan. B, an example of a force profile illustrating the force added when pulling Spm out of the WT filter towards the intracellular side. C, work profiles for pulling the three different polyamines to the intracellular side in the WT protein. Spm results are shown in black, Spd in orange and Put in cyan. The work involved in Spm release is generally larger than for release of the smaller polyamines. D, work profiles for pulling Spm to the intracellular side for the WT protein (black), the 2Pro mutant (orange) and the 4Pro mutant (cyan). E and H, example responses of GluK2/GluK5(P599G) (E, patch 130610p7) and GluK2(G615P) (H, patch 130606p2) at various holding potentials (−100 to +100 mV, 20 mV increments) in the presence of 60 μm internal spermine. Average I–V plots (F, I) and corrected G–V plots (G, J) for these receptors in the presence of internal spermine. Relationships for GluK2 and GluK2/GluK5 (grey lines in G and J) are shown for comparison. Data are represented as mean ± SEM. Current values are normalized to the current at −100 mV.
