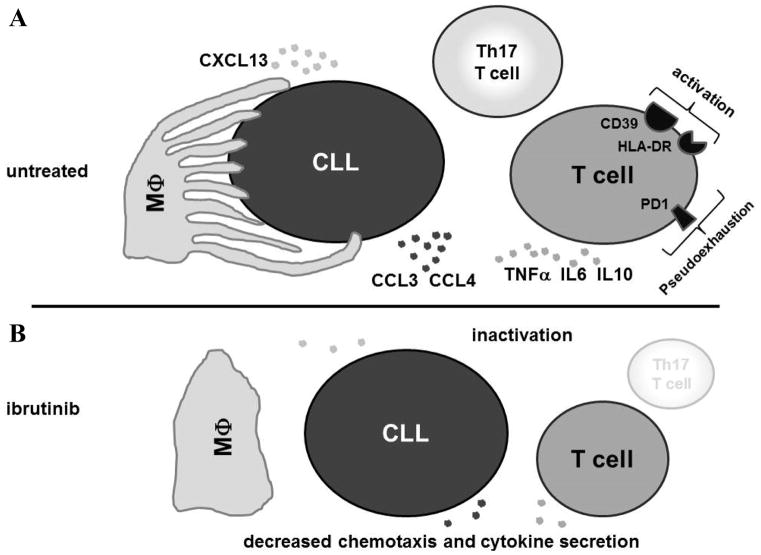
Figure 6. Changes in the tumor-microenvironment in CLL patients treated with ibrutinib.
A, CLL cells in patients before starting ibrutinib interact with T cells and macrophages (MΦ), and are surrounded by a multitude of soluble factors. Macrophages form extensions and maintain multiple sites of contact with the CLL cells and secrete CXCL13 that functions as a chemoattractant for CLL cells.(41, 49) Activated CLL cells in turn secrete CCL3 and CCL4 among other chemoattractants that stimulate macrophages and T cells.(10, 17, 37) T cells and macrophages secrete TNFα, IL8 and IL10 among other inflammatory cytokines.(11, 29) These cytokines in turn cause an inflammatory state and support CLL proliferation.(29, 50) T cells express markers of cellular activation like CD39, HLA-DR and PD-1, which has been linked to pseudoexhaustion and inhibition of immune surveillance.(13) The Th17 subset of T cells is expanded and implicated in the inflammatory drive in CLL and the development of autoimmune cytopenias.(14) B, On ibrutinib treatment, the tumor-microenvironment undergoes major changes. CLL cell activation, proliferation and cytokine/chemokine secretion is reduced.(22) In parallel, T cell activation and pseudoexhaustion is decreased, and T cell subsets shift with a marked reduction in Th17 cells. Cytokine and chemokine secretion from T cells and macrophages is decreased. Macrophage extensions detach from CLL cells and there is decreased CXCL13 secretion and consequently less migration of CLL cells. Taken together, the microenvironment on ibrutinib is reset from a hyperstimulated inflammatory state that supports tumor growth and inhibits anti-tumor T cell responses into a more resting state.