Abstract
Background
Chronic rhinosinusitis (CRS) and asthma describe inflammation of the upper and lower airway, respectively. Not surprisingly, the prevalence of CRS and asthma has been linked, with up to 50% asthma prevalence in CRS with nasal polyps (CRSwNP) patients. However, these prevalence rates do not address subtypes of CRSwNP including allergic fungal rhinosinusitis (AFRS). This study sets out to objectively determine asthma prevalence in CRS subtypes prospectively.
Methods
A prospective prevalence study of adult CRS patients was conducted over a 1 year period at a tertiary care center. Patients were grouped into CRSwNP, CRS without nasal polyps (CRSsNP), or AFRS. Patients were administered an Asthma Screening Questionnaire (ASQ) and confirmed by pulmonary function testing (PFT) if positive on the ASQ. Chi squared analysis was performed to compare the asthma prevalence among the CRS subtypes.
Results
A total of 410 patients (age 48.1 ± 16.4, 53.5% male) were included. Of these, 178 (43.4%) had CRSwNP, 166 (40.5%) had CRSsNP, and 66 (16.1%) met criteria for AFRS. Analysis revealed that 48.3% of CRSwNP patients, 16.5% of CRSsNP patients, and 23.6% of AFRS patients had asthma confirmed by PFTs. Chi squared analysis showed a significant difference in asthma prevalence between CRSwNP and AFRS (p=0.0016) and CRSwNP and CRSsNP (p=0.0000), but no significant difference between CRSsNP and AFRS (p=0.2380).
Conclusion
There is a significant difference in the prevalence of asthma between CRSwNP and AFRS, suggesting a fundamental distinction in their etiologies despite similar immunologic profiles. Further efforts to delineate these biological disparities are underway.
Keywords: Chronic rhinosinusitis, asthma, allergic fungal sinusitis, nasal polyps
Introduction
Chronic rhinosinusitis (CRS) is a condition that affects over 31 million people annually in the United States.1 It encompasses a spectrum of disorders involving inflammation of the paranasal sinuses and nasal passages resulting in facial pain and pressure, anosmia, and mucopurulent drainage. CRS manifests in various ways including CRS with nasal polyposis (CRSwNP), CRS without nasal polyposis (CRSsNP), and allergic fungal rhinosinusitis (AFRS). Numerous etiologies including bacteria, viruses, fungi, allergy, and anatomical variance have been proposed.2 CRS with nasal polyps is of particular interest as it represents a diagnosis with several clinical subsets, including AFRS, cystic fibrosis, aspirin exacerbated respiratory disease, and CRSwNP not otherwise specified.
Inflammation is the cornerstone of the pathophysiology of CRS. The concept of the unified airway suggests that upper airway inflammation may influence lower airway inflammation and vice versa.2 Asthma is an inflammatory condition of the lower airway causing variable expiratory obstruction resulting in episodic wheezing, dyspnea, and cough.3 Forty to 75% of all adults and children with asthma have concurrent rhinosinusitis.4
Recent evidence has shown that CRS and asthma share not only a physical link of the affected organs, but also biochemical, histological, and clinical characteristics. In Western countries, CRSwNP and allergic asthma share a type 2 inflammatory response, characterized by elevated levels of IL-4, IL-5, IL-13, and eosinophils. Recently, biomarkers such as nitric oxide and IL-17 have also been implicated in the pathogenesis of these two conditions.5 Clinically, increasing asthma severity has been associated with worsening radiological evidence of CRS in addition to higher prevalence of nasal polyposis and allergic sensitization.6 Medical and surgical treatment of sinusitis in patients with asthma has been shown to decrease asthmatic and sinonasal symptoms.7
Retrospective evaluation of our patients revealed that asthma was more prevalent in patients with CRSwNP as compared to patients with AFRS.8 However, asthma is a clinical diagnosis and is often not formally diagnosed with a pulmonary function test (PFT). In this study, we set out to objectively determine the prevalence of PFT-proven asthma in several CRS subtypes, CRSwNP, AFRS and CRSsNP.
Methods
Study Design
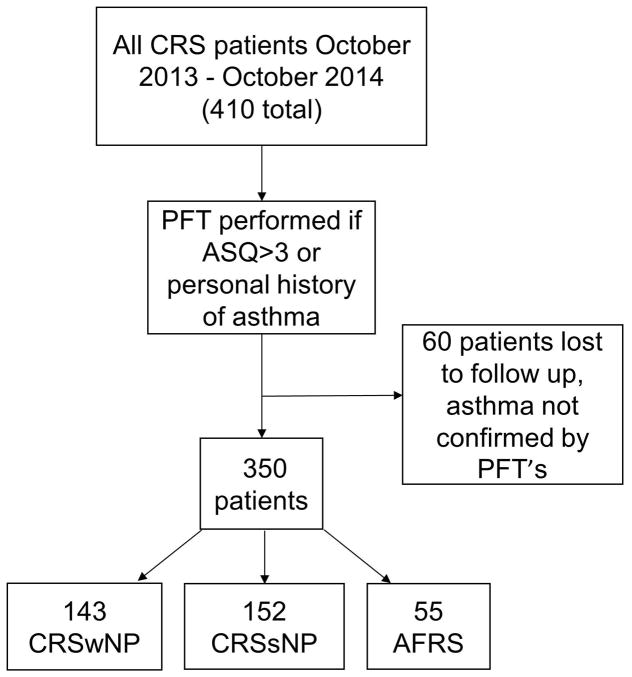
A prospective prevalence study of CRS patients was conducted over a 1 year period (October 2013-October 2014) at the University of Texas Medical School at Houston. The Institutional Review Board at the University of Texas Health Science Center at Houston approved the study protocol. All patients with CRS were administered an Asthma Screening Questionnaire (ASQ) developed by Shin et al.9 If the patient scored >3 on the ASQ and/or reported a history of asthma, PFT was performed. Patients who did not complete the ASQ or PFT testing when indicated were excluded from analysis (see Figure 1). Patients’ age, gender, current asthma status, CRS subtype, ASQ score, and PFT results were recorded (see Table 1).
Figure 1. Workflow of Patients Included in Asthma Prevalence Analysis.
Four hundred and ten new and established patients with chronic rhinosinusitis seen in the ENT clinic between October 2013 - October 2014 comprised the initial cohort. This population was screened and underwent PFT as indicated to calculate the number of patients with asthma in each CRS subtype.
Table 1.
Demographics depicting various characteristics among CRS subtypes
| Mean Age (Age Range) | %Male | Average ASQ | Asthma Prevalence | |
|---|---|---|---|---|
| CRSwNP | 50.4 (16–81) | 57.3% | 5.3 | 48.3% |
| CRSsNP | 50.5 (19–85) | 49.4% | 2.6 | 16.4% |
| AFRS | 36.7 (16–75) | 50.0% | 2.8 | 23.6% |
Diagnosis and Classification
Patients were grouped into CRSwNP, CRSsNP, or AFRS according to criteria set forth in the European Position Paper on Rhinosinusitis and Nasal Polyps.10 Patients were grouped into CRSwNP or CRSsNP based on presence or absence of polyps on nasal endoscopy. Polyps documented by an ENT physician on nasal endoscopy at any time categorized a patient as CRSwNP subtype. CRSwNP patients were diagnosed with AFRS if there was evidence or documented history of eosinophilic mucin with noninvasive fungal hyphae, hypersensitivity to fungi, and characteristic radiographic findings.11
Statistical Analysis
Demographic and clinical characteristics of CRS patients were tabulated. Chi squared analysis was performed comparing the asthma prevalence among the CRS subtypes. Kruskal-Wallis rank test was used to determine if there was a significant difference in ASQ scores among the groups.
Results
A total of 410 patients (age 48.1 ± 16.4, 53.5% male) were included. Of these, 178 (43.4%) had CRSwNP, 166 (40.5%) had CRSsNP, and 66 (16.1%) met criteria for AFRS. Thirty-five of 178 (19.7%) CRSwNP patients, 14 of 166 (8.43%) CRSsNP patients, and 11 of 66 (16.67%) AFRS patients either did not answer the ASQ or did not perform PFT’s when indicated for this study and therefore were excluded from the analysis.
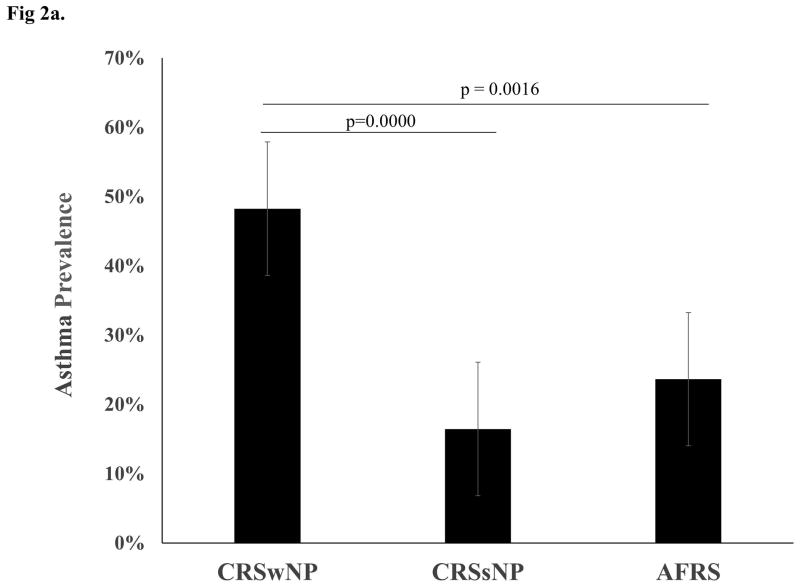
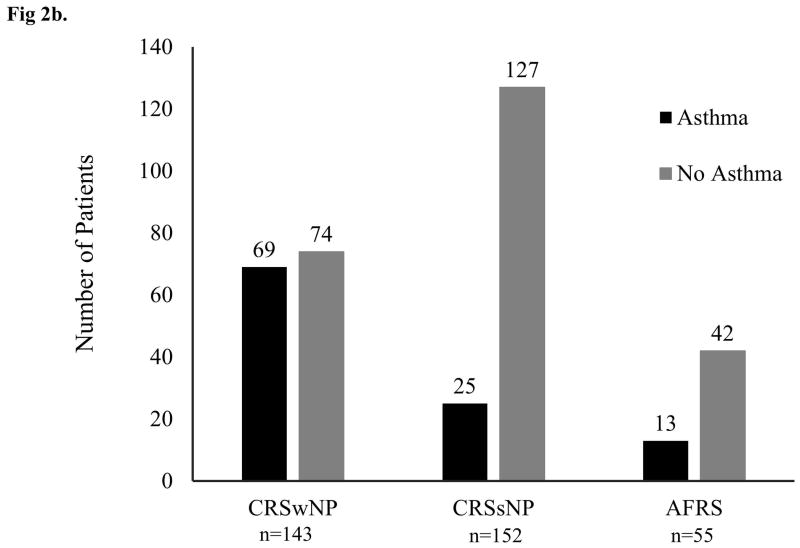
Of the 350 patients available for analysis, 48.3% of CRSwNP patients, 16.5% of CRSsNP patients, and 23.6% of AFRS patients had asthma confirmed by PFTs. Chi squared analysis showed a significant difference in asthma prevalence between CRSwNP and AFRS (p=0.0016) and CRSwNP and CRSsNP (p=0.0000), but no significant difference between CRSsNP and AFRS (p=0.2380) (see Figure 2). Average ASQ scores for patients with CRSwNP, CRSsNP, and AFRS were 5.3, 2.6, and 2.8, respectively. Kruskal-Wallis rank test showed a significance in ASQ scores among the CRS subtypes (p=0.0001). Wilcoxon rank-sum test was used to compare the groups and revealed a significant difference in ASQ scores between CRSwNP and CRSsNP (p=0.0001) and between CRSwNP and AFRS (0.0022), but no significant difference between CRSsNP and AFRS (p=0.1765).
Figure 2. Asthma Prevalence in CRS Subtypes.
(a) Graph shows prevalence of asthma in each of the three CRS subtypes with standard error bars. P-values between groups represent the outcome of Chi-squared analysis. (b) Graph depicts the number of patients with PFT-proven asthma vs. no disease in each of the three CRS subtypes studied.
Discussion
In this study, we found a significantly higher prevalence of confirmed asthma of 48.3% in CRSwNP as compared to a clinical CRSwNP subtype, AFRS, in which asthma prevalence was 23.6%. As expected, there was also a significantly higher asthma prevalence in patients with CRSwNP as compared to patients with CRSsNP in which asthma prevalence was 16.5%. Patients with CRSwNP also had significantly higher ASQ scores compared to patients with CRSsNP and AFRS. This is the largest prospective study evaluating the prevalence of asthma in CRS subtypes in which asthma was confirmed with objective testing.
Classically, CRSwNP and AFRS are characterized by a type 2 inflammatory disease with associated eosinophilic inflammation and edema whereas CRSsNP is a neutrophilic, type 1 predominant response with increased expression of transforming growth factor (TGF) β1 and collagen.12,13 Like CRSwNP and AFRS, allergic asthma is associated with eosinophilia and a type 2 inflammatory response suggesting a common pathogenesis.5 Studies show eosinophilia correlates with greater severity of sinus disease as noted by CT score, mucosal damage, clinical symptoms, and upper airway remodeling.14,15
Given overlapping similarities in the immunologic profile between CRSwNP, AFRS, and asthma, it was surprising to find significant differences in asthma prevalence rates between CRSwNP and AFRS. The 48.3% prevalence of asthma in CRSwNP patients is consistent with levels reported in the literature.16,17,18 Although not specifically studied, Clark et al also found similarly significantly lower percentage of asthma in AFRS patients as compared to non-AFRS CRSwNP patients at 23.5%.8 The present study showed a significant difference in the asthma prevalence rates between AFRS and non-AFRS CRSwNP patients. Other than the similar type 2 inflammatory changes within the affected sinonasal mucosa, AFRS is unique from other CRSwNP patients in that it is defined by sinuses obstructed by fungal laden eosinophilic mucin and IgE-mediated hypersensitivity to fungi.11,19 These clinical differences and now the significant differences in the prevalence rates of asthma between AFRS and other CRSwNP patients suggest a distinct biological mechanism responsible for AFRS.
Recent studies have shown that fungi can incite airway sensitization through activation of innate and adaptive immune responses, leading to inflammation and tissue injury.2 Upon immunologic challenge with fungi, respiratory epithelial cells can release IL-25, IL-33, and thymic stromal lymphopoietin which initiate an innate and adaptive type 2 inflammatory response.20 Mucosal reaction to locally produced fungal proteases can also cause a type 2 mediated response.21 Another study showed that an Aspergillus serine protease promoted airway hyper-responsiveness by mucosal infiltration.22 These studies link fungi to the pathophysiology of asthma and provide molecular insight into how fungi can incite a type 2 inflammatory response in the airway.
Recently, Porter et al demonstrated a link between fungi and CRS and specifically in patients with CRSwNP and AFRS. In their study, they found increased presence of fungi in the sinus cavity of CRSwNP and AFRS patients as compared to patients without CRSsNP and non-CRS controls. In addition, they demonstrated using an ELISpot assay that CRSwNP and AFRS patients had immune memory to the fungi found in their sinus cavities. In those few non-CRS patients where fungi was identified, they had not mounted an immune response to the fungi.23 These evidence support how fungal exposure and sensitivity can contribute to the pathophysiology of CRSwNP and increase the prevalence of asthma in CRSwNP patients as compared to the general population, yet the observed difference in asthma prevalence between AFRS and CRSwNP is a mystery.
Perhaps the unique clinical and pathologic findings of AFRS are due in part to immune dysfunction. Millien et al showed that in wild type mice, challenge with intranasal Aspergillus fungal proteinase resulted in classic features of allergic asthma including airway hyper-responsiveness, eosinophilic response, and mucin production. The study describes fungal protease acting on local fibrinogen releasing fibrinogen cleavage products. These products are known ligands of Toll-like receptor 4 (TLR4), an innate microbial receptor found on respiratory epithelial cells and innate immune cells such as macrophages. However, in TLR4−/− mice, this same intranasal fungal challenge resulted in attenuation of airway hyper-responsiveness, but an unaffected adaptive T helper 2 inflammatory response.24 This fungal protease activated TLR4-mediated pathway seems to affect the development of lower airway hyperreactivity (asthma) and innate immune response to airway fungi, but has minimal if any effect on the activation of the adaptive immune response characterized by lymphocytes and production of immunoglobulins. It is a defect in this pathway that may explain the lower prevalence of asthma, the inability to clear fungi from the sinus cavities due to failure to activate the innate response to fungi, and the typically elevated local and serum IgE levels in AFRS. Future work in our lab is exploring this possible molecular defect in the fungal protease activated TLR4-mediated pathway in AFRS.
Of course, there may be other possible molecular causes for the observed differential prevalence of asthma between AFRS and CRSwNP patient that have yet to be identified. These differences may also explain the differences in clinical response to typical treatment such as surgery and steroids. In addition, the difference in typical age of presentation between AFRS and CRSwNP may have an impact on asthma development. Our study did not have enough patients to explore effects of age and timing of asthma diagnosis relative to development of sinus disease to explore those possibilities. Clearly, additional studies are needed to identify molecular differences between AFRS and CRSwNP.
The significantly higher asthma prevalence in CRSwNP patients as compared to CRSsNP was expected. Our prevalence of asthma of 16.5% is comparable to rates reported in the literature of 22% for CRSsNP16. Tissue from CRSsNP patients are typically characterized by prominent TGF-β and IFN-γ levels instead of type 2 cytokines such as IL-5 as found in CRSwNP.25 These differences in immunologic profile between CRS without nasal polyps and CRSwNP and asthma suggest they have disparate etiologic pathways and may explain the lower asthma prevalence in patients with CRSsNP.
These novel findings have implications pathophysiology and treatment of the CRS and concurrent asthma. Studies have shown that medical and surgical treatment of rhinosinusitis in patients with asthma not only improves sinonasal and asthma symptoms, but also decreases physician visits and the need for medication.7 Endoscopic sinus surgery improved sinus and asthma symptoms and decreased glucocorticoid requirements in children with severe asthma and CT evidence of chronic sinus disease.26 Moreover, systemic anti-inflammatory agents such as prednisone, montelukast, and omalizumab can improve subjective and objective nasal and pulmonary symptoms in patients with CRSwNP and asthma.27
As the complexity of the link between CRS and asthma and the pathophysiology of the CRS subtypes becomes appreciated, future treatments can be tailored thereby improving patients’ outcomes. One such example is the use of antifungals. Given the possibility that a defect in a fungal protease activated TLR4-mediated pathway may explain the inability to clear fungi from diseased sinuses of AFRS, antifungals may be a more effective treatment option in AFRS as compared to other CRS subtypes.
In this study, we found significant differences in the prevalence of asthma between CRSwNP and AFRS despite these two CRS subtypes sharing similar type 2 inflammatory response, and significant differences in CRSwNP and CRSsNP as reported in the literature.
Conclusion
CRS is a disease that has long been associated with asthma. In this study, we found a significantly higher prevalence of asthma and significantly higher asthma scores in patients with CRSwNP when compared to patients with CRSsNP and AFRS. This observation suggests differences in the pathophysiology of CRS subtypes, even in subtypes that otherwise share similar immunologic and clinical characteristics. As work in our lab and others continues to elucidate the relation between these two comorbid conditions, specific treatments may need to be modified to combat the unique molecular pathogenesis among the CRS subtypes in order to improve patients’ quality of lives.
Acknowledgments
Financial Support: This study was supported by a Foundation grant from the American Academy of Otolaryngic Allergy. AL is supported by the Center for Clinical and Translational Sciences, which is funded by National Institutes of Health Clinical and Translational Award UL1 TR000371 and KL2 TR000370 from the National Center for Advancing Translational Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Science or the National Institutes of Health.
We would like to thank Dr. Drew Plonk, Jackie Sherfield and Jose Elias for their clinical support in assisting with administering questionnaires and performing pulmonary function tests.
Footnotes
Authorship: AL conceived the study and its design with assistance from MJC, SF and PP. AL, MJC and SF implemented the analysis with assistance from CP and SK. SK and AL wrote the manuscript, and CK, MJC, SF and PP edited the manuscript and provided conceptual advice.
Conflict of Interest: AL received consulting fees from GREER and ENTvantage and industry research funding from IntersectENT, Amgen and Mallinckrodt. MJC received consulting fees from JNJ/Acclarent and Polyganics. SF received consulting fees from IntersectENT.
References
- 1.Infectious rhinosinusitis in adults: classification, etiology and management. International Rhinosinusitis Advisory Board. Ear Nose Throat J. 1997;76:1–22. [PubMed] [Google Scholar]
- 2.Meltzer EO, et al. Rhinosinusitis: developing guidance for clinical trials. J Allergy Clin Immunol. 2006;118:S17–61. doi: 10.1016/j.jaci.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet Lond Engl. 2010;376:803–813. doi: 10.1016/S0140-6736(10)61087-2. [DOI] [PubMed] [Google Scholar]
- 4.Spector SL, et al. Parameters for the diagnosis and management of sinusitis. J Allergy Clin Immunol. 1998;102:S107–144. doi: 10.1016/s0091-6749(98)70045-4. [DOI] [PubMed] [Google Scholar]
- 5.Pakdaman MN, Luong A. The links between chronic rhinosinusitis and asthma. Curr Opin Otolaryngol Head Neck Surg. 2011;19:218–223. doi: 10.1097/MOO.0b013e32834500a8. [DOI] [PubMed] [Google Scholar]
- 6.Lin DC, et al. Association between severity of asthma and degree of chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25:205–208. doi: 10.2500/ajra.2011.25.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senior BA, Kennedy DW. Management of sinusitis in the asthmatic patient. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 1996;77:6–15. doi: 10.1016/S1081-1206(10)63473-4. quiz 15–19. [DOI] [PubMed] [Google Scholar]
- 8.Clark DW, Wenaas A, Luong A, Citardi MJ, Fakhri S. Staphylococcus aureus prevalence in allergic fungal rhinosinusitis vs other subsets of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2013;3:89–93. doi: 10.1002/alr.21090. [DOI] [PubMed] [Google Scholar]
- 9.Shin B, Cole SL, Park SJ, Ledford DK, Lockey RF. A new symptom-based questionnaire for predicting the presence of asthma. J Investig Allergol Clin Immunol. 2010;20:27–34. [PubMed] [Google Scholar]
- 10.Fokkens WJ, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;3:1–298. p preceding table of contents. [PubMed] [Google Scholar]
- 11.Bent JP, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 1994;111:580–588. doi: 10.1177/019459989411100508. [DOI] [PubMed] [Google Scholar]
- 12.Van Zele T, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 13.Van Bruaene N, et al. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:253–259. 259.e1–2. doi: 10.1016/j.jaci.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Brinke A ten, et al. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J Allergy Clin Immunol. 2002;109:621–626. doi: 10.1067/mai.2002.122458. [DOI] [PubMed] [Google Scholar]
- 15.Barham HP, et al. Remodeling changes of the upper airway with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;5:565–572. doi: 10.1002/alr.21546. [DOI] [PubMed] [Google Scholar]
- 16.Batra PS, Tong L, Citardi MJ. Analysis of comorbidities and objective parameters in refractory chronic rhinosinusitis. The Laryngoscope. 2013;123(Suppl 7):S1–11. doi: 10.1002/lary.24418. [DOI] [PubMed] [Google Scholar]
- 17.Staikūniene J, Vaitkus S, Japertiene LM, Ryskiene S. Association of chronic rhinosinusitis with nasal polyps and asthma: clinical and radiological features, allergy and inflammation markers. Med Kaunas Lith. 2008;44:257–265. [PubMed] [Google Scholar]
- 18.Håkansson K, et al. A comparative and descriptive study of asthma in chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2014;28:383–387. doi: 10.2500/ajra.2014.28.4076. [DOI] [PubMed] [Google Scholar]
- 19.Luong A, Davis LS, Marple BF. Peripheral blood mononuclear cells from allergic fungal rhinosinusitis adults express a Th2 cytokine response to fungal antigens. Am J Rhinol Allergy. 2009;23:281–287. doi: 10.2500/ajra.2009.23.3311. [DOI] [PubMed] [Google Scholar]
- 20.Plonk DP, Luong A. Current understanding of allergic fungal rhinosinusitis and treatment implications. Curr Opin Otolaryngol Head Neck Surg. 2014;22:221–226. doi: 10.1097/MOO.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 21.Pakdaman MN, Corry DB, Luong A. Fungi linking the pathophysiology of chronic rhinosinusitis with nasal polyps and allergic asthma. Immunol Invest. 2011;40:767–785. doi: 10.3109/08820139.2011.596876. [DOI] [PubMed] [Google Scholar]
- 22.Balenga NA, et al. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat Commun. 2015;6:6763. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter PC, et al. Airway surface mycosis in chronic TH2-associated airway disease. J Allergy Clin Immunol. 2014;134:325–331. doi: 10.1016/j.jaci.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millien VO, et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013;341:792–796. doi: 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, et al. Differential Expression and Release of Activin A and Follistatin in Chronic Rhinosinusitis with and without Nasal Polyps. PloS One. 2015;10:e0128564. doi: 10.1371/journal.pone.0128564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manning SC, Wasserman RL, Silver R, Phillips DL. Results of endoscopic sinus surgery in pediatric patients with chronic sinusitis and asthma. Arch Otolaryngol Head Neck Surg. 1994;120:1142–1145. doi: 10.1001/archotol.1994.01880340082014. [DOI] [PubMed] [Google Scholar]
- 27.Rix I, Håkansson K, Larsen CG, Frendø M, von Buchwald C. Management of chronic rhinosinusitis with nasal polyps and coexisting asthma: A systematic review. Am J Rhinol Allergy. 2015;29:193–201. doi: 10.2500/ajra.2015.29.4178. [DOI] [PubMed] [Google Scholar]