Figure 1.
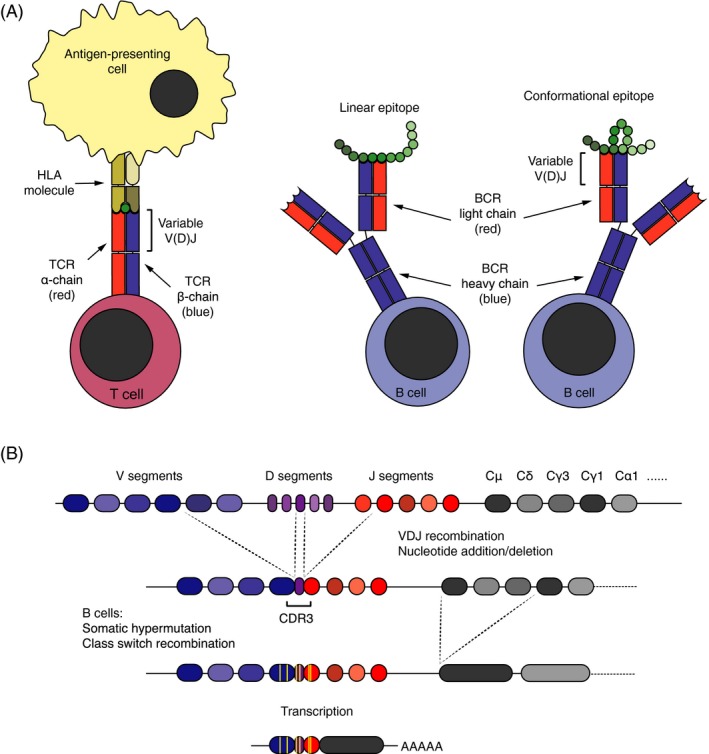
Structure, function, and diversification of antigen receptors. (A) The T‐cell receptor (TCR) binds to linear epitopes presented on HLA molecules by antigen‐presenting cells. The B‐cell receptor (BCR), in contrast, recognizes linear or conformational epitopes on native antigens. Both antigen receptors are composed of distinct pair of chains: The TCR of an α‐ and a β‐chain, and the BCR of two heavy and two light chains. The variable part of the receptor chains, encoded by V, J, and D (TCR β and BCR heavy) gene segments, constitutes their antigen‐binding surface. (B) During T‐ and B‐cell development, the V, J, and D (TCR β and BCR heavy) gene segments are stochastically recombined, and nucleotides may also be randomly added and deleted between them. The diversity of the receptor genes concentrates in the third complementarity determining region (CDR3), which encodes the center of the antigen‐binding surface. In the course of antigen‐driven immune responses, B cells may go through an additional round of diversification in germinal centers, where they undergo somatic hypermutation and clonal selection. During this process, they also switch the isotype of the constant chain. Class switch recombination leading to isotype switching from IgM (encoded by Cμ gene segments) to immunoglobulin G (IgG)1 (encoded by Cγ1 gene segments) is depicted.
