Figure 2. Yippee‐like globular domains of Mis18 proteins possess an intrinsic preference to form homo/heterodimers.
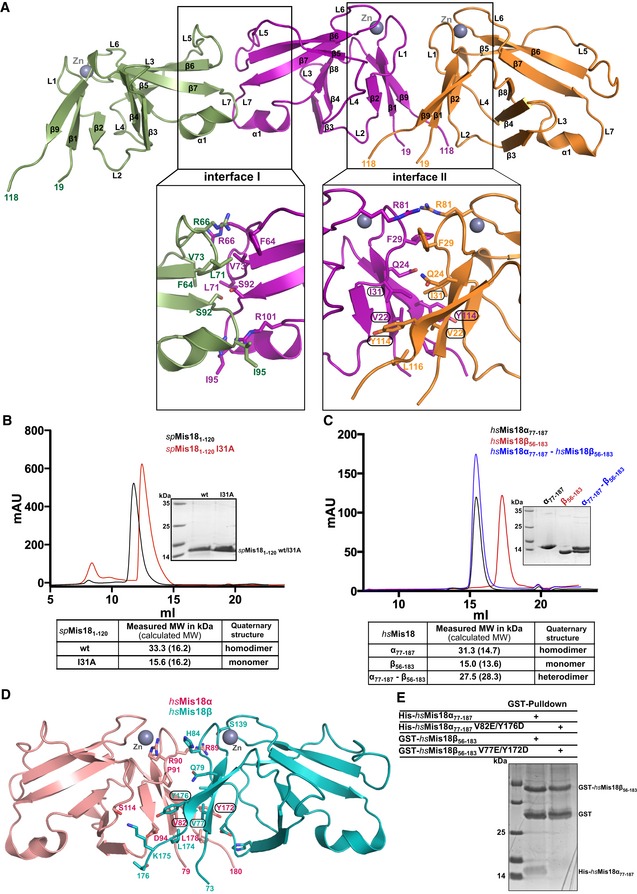
- Cartoon representation of the assembly of spMis18 Yippee‐like domains (spMis181–120) as observed in the crystal asymmetric unit. Close‐up views highlight the amino acid composition and interactions stabilizing the dimeric arrangements. Interface I is composed of conserved residues and an extensive binding surface as compared to interface I (see text for details), suggesting dimer II to be a physiologically relevant dimer. Residues mutated in this study are highlighted with circles.
- Size‐exclusion chromatography (SEC) and SEC‐MALS (SEC combined with multi‐angle light scattering) analyses of the recombinant wild‐type (wt) and dimer‐disrupting mutant (I31A) of spMis181–120. Measured molecular weights from SEC‐MALS confirm that spMis181–120 wt is a dimer in solution and spMis181–120I31A is a monomer.
- Evaluation of the ability of Yippee‐like domains of human Mis18 proteins (hsMis18α77–187 and hsMis18β56–183) to form a heterodimer. SEC and SEC‐MALS analyses demonstrate that while hsMis18α77–187 elutes at 15.5 ml as a dimer (31.3 kDa), hsMis18β56–183 elutes at 17.3 ml as a monomer (15.0 kDa). Purified hsMis18α77–187 and hsMis18β56–183 when mixed together elute at 15.4 ml as a heterodimer (27.5 kDa).
- Cartoon representation of the structure of the homology‐modeled human Mis18α77–187–Mis18β56–183 heterodimer using the crystal structure of spMis181–120 reported here as a template. Residues mutated in this study are highlighted with circles. Modeling was carried out using Phyre2 web server (www.sbg.bio.ic.ac.uk/phyre2/).
- SDS–PAGE analysis of the GST pull‐down assay where wt and dimer‐disrupting mutants of hsMis18α77–187 and hsMis18β56–183 were co‐expressed as His‐ and GST‐tagged proteins, respectively, in E. coli. While wt GST‐hsMis18β56–183 showed interaction with wt His‐hsMis18α77–187, hsMis18 proteins harboring dimer‐disrupting mutations, GST‐hsMis18β56–183V77E/Y172D, and His‐hsMis18α77–187V82E/Y176D did not show noticeable interaction. The corresponding Ni‐NTA pull‐downs showing the expression of His‐tagged proteins are shown in Fig EV2D.
