Abstract
Background:
The interaction between activated microglia and T lymphocytes can yield abundant pro-inflammatory cytokines. Our previous study proved that thymus immune tolerance could alleviate the inflammatory response. This study aimed to investigate whether intrathymic injection of myelin basic protein (MBP) in mice could suppress the inflammatory response after co-culture of T lymphocytes and BV-2 microglia cells.
Methods:
Totally, 72 male C57BL/6 mice were randomly assigned to three groups (n = 24 in each): Group A: intrathymic injection of 100 μl MBP (1 mg/ml); Group B: intrathymic injection of 100 μl phosphate-buffered saline (PBS); and Group C: sham operation group. Every eight mice in each group were sacrificed to obtain the spleen at postoperative days 3, 7, and 14, respectively. T lymphocytes those were extracted and purified from the spleens were then co-cultured with activated BV-2 microglia cells at a proportion of 1:2 in the medium containing MBP for 3 days. After identified the T lymphocytes by CD3, surface antigens of T lymphocytes (CD4, CD8, CD152, and CD154) and BV-2 microglia cells (CD45 and CD54) were detected by flow cytometry. The expressions of pro-inflammatory factors of BV-2 microglia cells (interleukin [IL]-1β, tumor necrosis factor-α [TNF-α], and inducible nitric oxide synthase [iNOS]) were detected by quantitative real-time polymerase chain reaction (PCR). One-way analysis of variance (ANOVA) and the least significant difference test were used for data analysis.
Results:
The levels of CD152 in Group A showed an upward trend from the 3rd to 7th day, with a downward trend from the 7th to 14th day (20.12 ± 0.71%, 30.71 ± 1.14%, 13.50 ± 0.71% at postoperative days 3, 7, and 14, respectively, P < 0.05). The levels of CD154 in Group A showed a downward trend from the 3rd to 7th day, with an upward trend from the 7th to 14th day (10.00 ± 0.23%, 5.28 ± 0.69%, 14.67 ± 2.71% at postoperative days 3, 7, and 14, respectively, P < 0.05). The ratio of CD4+/CD8 + T in Group A showed a downward trend from the 3rd to 7th day, with the minimum at postoperative day 7, then an upward trend from the 7th to 14th day (P < 0.05). Meanwhile, the levels of CD45 and CD54 in Group A were found as the same trend as the ratio of CD4+/CD8 + T (CD45: 83.39 ± 2.56%, 82.74 ± 2.09%, 87.56 ± 2.11%; CD54: 3.80 ± 0.24%, 0.94 ± 0.40%, 3.41 ± 0.33% at postoperative days 3, 7, and 14, respectively, P < 0.05). The expressions of TNF-α, IL-1β, and iNOS in Group A were significantly lower than those in Groups B and C, and the values at postoperative day 7 were the lowest compared with those at postoperative days 3 and 14 (P < 0.05). No significant difference was found between Groups B and C.
Conclusions:
Intrathymic injection of MBP could suppress the immune reaction that might reduce the secondary immune injury of brain tissue induced by an inflammatory response.
Keywords: Brain Injuries, Immune Inflammation, Intrathymic Injection, Myelin Basic Protein
INTRODUCTION
Increasingly available evidence showed that autoimmune attack plays a critical role in brain damage, even in cerebral ischemic injury.[1] The autoantigens, including neuron specific enolase (NSE), S100 calcium-binding protein β (S-100β) protein, myelin basic protein (MBP), and etc., exist in “immunological privileged site”[2] normally. However, when a blood-brain barrier (BBB) is broken, the autoantigens would leak into peripheral circulation and activate T lymphocytes.[3,4] Besides, the resident immune cells, microglia, for example, could induce autoimmune reaction by presenting autoantigen to T lymphocytes.[5] Researchers[6,7,8] showed that the interaction between activated microglia and T lymphocytes can yield abundant pro-inflammatory cytokines. Benson et al.[9] and Meyer et al.[10] found that the clinical symptoms of relapsing experimental autoimmune encephalomyelitis could be reduced by oral administration of MBP. Ayer et al.[11] successfully induced immune tolerance through instilling autoantigen to the nasal mucosa, and found that it had a neuroprotective effect on brain injury. Our previous study[12] proved that thymus immune tolerance induced by cerebrospinal fluid injection could change the level of T-lymphocyte and alleviate the inflammatory response. In this study, we aimed to investigate whether intrathymic injection of MBP could have the same effect and whether it could down-regulate the inflammation response in the central nervous system by suppressing the activity of microglia.
METHODS
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Tianjin Public Health Bureau.
Animals and grouping
Male C57BL/6 mice, 6–8 weeks old, were offered by Tianjin AoYi Experimental Animal Breeding Co., Ltd., China (SCXK-2012-0004). The mice were housed in a pathogen-free facility and given ample food and water. The environment temperature was 20–25°C, and relative wet degree was 55%–70%. High-purity MBP (Enzo Life Sciences, NY, USA) derived from mice brain was emulsified in equivalent complete Freund's adjuvant (Sigma, MO, USA). Totally, 72 mice were randomly assigned to three groups (n = 24 in each): Group A: intrathymic injection of 100 μl MBP (1 mg/ml); Group B: intrathymic injection of 100 μl phosphate-buffered saline (PBS); and Group C: sham operation group.
Procedure of intrathymic injection
The mice were fasted 12 h before the experiment with freely drinking and then were anesthetized with chloral hydrate (0.3 ml/100 g) by intraperitoneal injection. The animals were fixed on the surgery shelves in the supine position. After the skin preservation and sterilization, a median incision was made, the anterior tip of sternum bone was removed, muscles were separated bluntly, and the pink and white thymuses were exposed. Then, 100 μl MBP (50 μl in each side) or 100 μl PBS were injected into the thymuses in the mice of Groups A and B, respectively, while the mice in Group C were just punctured on both sides of the thymus with nothing injected. After the intrathymic injection, the mice were sutured layer by layer, disinfected, and warped up.
Extraction of T lymphocytes
Every eight mice in each group were sacrificed to obtain the spleen according to the method in Gottrand et al.'s study[13] at postoperative days 3, 7, and 14, respectively. Before the sacrificion, nylon fiber column (Polysciences, PA, USA) was incubated for 1 h using 1640 culture medium containing 10% fetal bovine serum (FBS) (Gibco, MA, USA). The mice were sacrificed by carbon dioxide inhalation and then immersed in 75% alcohol for 10 min to be sterilized. Their spleen was obtained and grinded on 200-mesh stencil aseptically. Grinding fluid was centrifuged in 900 rpm for 5 min, the supernatant was abandoned, and 2 ml 1X red blood cell lysis buffer was added. The solution was then centrifuged again, the supernatant was abandoned, the deposit was resuspended by PBS, and then, cells were collected into the culture flask after transiting nylon fiber columns.
Culture of BV-2 microglia cells
The gene-modified BV-2 microglia cells were highly purified. BV-2 cell line was chosen in this study due to the same features as primary microglia including morphology, phenotypes, and functions, and its facility to cultivate. BV-2 cells were cultured in 10% complete Dulbecco's minimum Essential medium (Gibco, MA, USA) containing 10% FBS and 1% penicillin-streptomycin (Biological Industries, KBH, Israel).
Co-culture of BV-2 cells and T lymphocytes
Interferon-γ (IFN-γ) (PeproTech, NJ, USA) (100 U/ml) and granulocyte macrophage-colony stimulating factor (GM-CSF) (PeproTech, NJ, USA) (10 ng/ml) were added to BV-2 cell culture medium to activate BV-2 cells for 7 days before co-culture.
Isolated T-cells were added to flasks which stayed in horizontal position for 3 h. Then, the flasks were stood upright in order to wipe off monocytes. Interleukin (IL)-2 and ConA were needed in co-culture of BV-2 cells and T-cells. Afterward, T-cells were added to the glial culture medium containing MBP in the proportion of 1:2 (0.5 × 105 T-cells: 1 × 105 BV-2 cells) [Figure 1].[14]
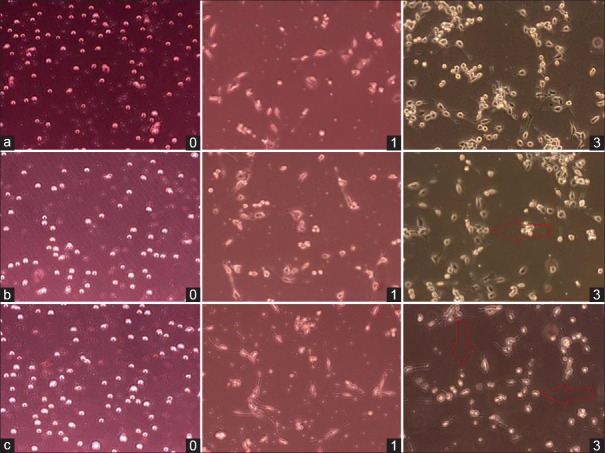
Figure 1.
Co-culture of T cells and BV-2 cells in Groups a–c at postoperative days 0, 1, and 3 under microscope (Original magnification ×10). Microglia cells were larger than T cells in morphology. The initial ratio of co-cultured T cells and BV-2 cells is 1:2 (day 0). After 1 day, partial microglia started the adherent growth and stretched out the synapse, especially performed in Group c; meanwhile, the suspended growing T lymphocytes got close to microglia. On the 3rd day, T cells went closer to microglia and some microglia even changed their cellular morphology into amoeba appearance (red arrow). Group a: Intrathymic injection of 100 μl myelin basic protein (MBP,1 mg/ml); Group b: Intrathymic injection of 100 μl phosphate buffered saline (PBS); Group c: Sham operation group.
Detection of surface antigens by flow cytometry
The antibodies were offered by BD Biosciences (CA, USA). Anti-CD45, anti-CD54 antibody, and the isotype controls were used to mark the BV-2 cells; after identified T lymphocytes by anti- CD3, the antibodies including anti-CD4, anti-CD8, anti-CD152, anti-CD154, and their isotype controls were applied to T-cells.
Quantitative real-time polymerase chain reaction (PCR) for pro-inflammatory factors
The expressions of pro-inflammation genes of BV-2 microglia cells, including tumor necrosis factor-α (TNF)-α, IL-1β, and inducible nitric oxide synthase (iNOS), were detected by quantitative real-time PCR.
M-MLV kit was bought from Life Technology Inc., (USA), regarding microglia RNA as template reverse transcription into complementary DNA. The primers (Sigma, USA) used were as follows:
TNF-α: Upstream primer 5′-CATCTTCTCAAAA TTCGAGTGACAA-3AT downstream 5′-TGGGAGTAGACAAGGTACAACCC-3G
IL-1β: Upstream primer 5′-CAACCAACAAGT GATATTCTCCATG-3AA downstream 5′-GATCCACACTCTCCAGCTGCA-3A
iNOS: Upstream primer 5′-CAGCTGGGCT GTACAAACCTT-3AG downstream 5′-CATTGGAAGTGAAGCGTTTCG-3A.
Statistical analysis
Quantitative data were showed as a mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to compare variables among the groups, and the least significant difference test was used for paired comparisons with the software SPSS 17.0 (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
Purity of extracted T lymphocytes from spleen
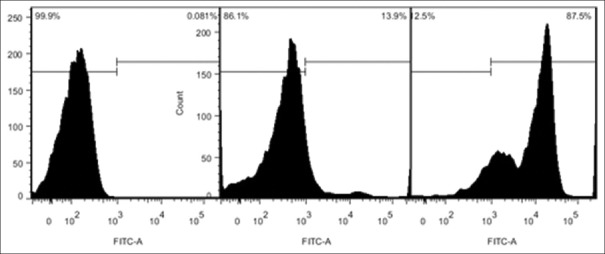
The flow cytometry showed that the mean percentage of T lymphocytes extracted from spleen could reach 85.0 ± 5.0%, the ratio of CD4+/CD8+ T could approximately reach 1.30 ± 0.15 [Figures 2 and 3]. The extract efficiency of T lymphocytes by nylon fiber column was high and stable enough for the co-culture with BV-2 cell in next step.
Figure 2.
Purity of T-cells derived from spleen attained to 85.0 ± 5.0% which could meet the experimental requirements (the axis indicate the number of positive cells).
Figure 3.
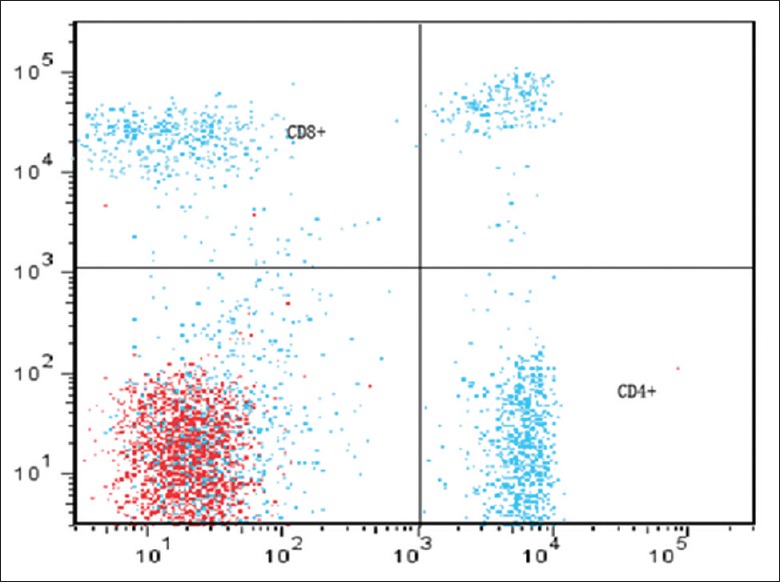
Ratio of CD4+/CD8+ T after transiting nylon fiber column. The ratio of CD4+/CD8+ T attained to 1.30 ± 0.15 before intrathymic injection of myelin basic protein (CD4 was labeled by PerCP-CY5, CD8 was labeled by PE-CY7) (The axis indicate the number of the positive cell).
Surface antigens in T-cells
Ratio of CD4+/CD8+ T
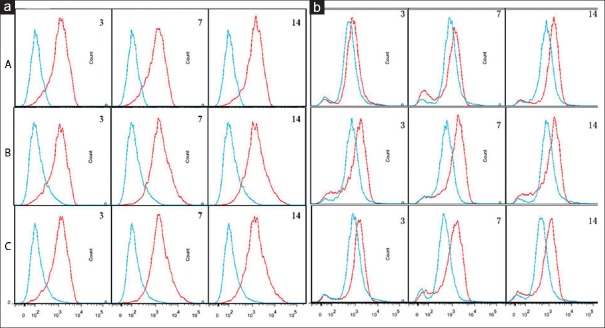
No significant difference could be found among the ratios in the three groups at postoperative days 3 and 14. However, the ratio in Group A showed a downward trend from the 3rd to 7th day, with the minimum at postoperative day 7, then an upward trend from the 7th to 14th day (P < 0.05) [Figure 4]. Besides, the ratios in both Groups B and C showed an upward trend from the 3rd to 7th day, with the maximum at postoperative day 7, then a downward trend from the 7th to 14th day (P < 0.05).
Figure 4.
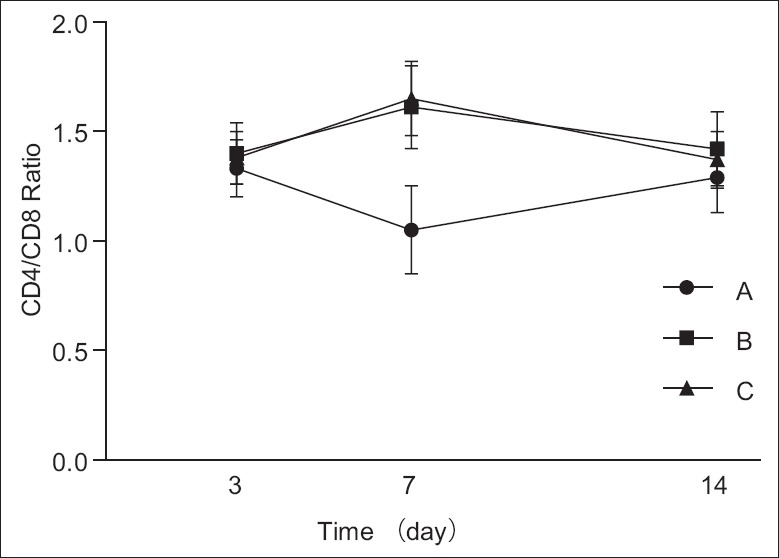
Ratio of three groups was almost the same on the 3rd and 14th day. As time passed, Group A showed a trend of decline, then ascended later. On the 7th day, the ratio declined to bottom. Group B and C increased first and then decreased. The ratio climbed to the climax on the 7th day. The difference was statistically significant on the 7th day (P < 0.05). Group A, intrathymic injection of 100 μl myelin basic protein (1 mg/ml); Group B: Intrathymic injection of 100 μl phosphate buffered saline (PBS); Group C: Sham operation group.
Levels of CD152 and CD154
The levels of CD152 in Group A at postoperative days 3 and 7 were obviously higher than those in Groups B and C, while no significant difference could be found among values in the three groups at postoperative day 14. In addition, the levels of CD152 in Group A showed an upward trend from the 3rd to 7th day, with a downward trend from the 7th to 14th day (20.12 ± 0.71%, 30.71 ± 1.14%, and 13.50 ± 0.71% at postoperative days 3, 7, and 14, respectively, P < 0.05). No significant difference could be found between Groups B and C [Table 1].
Table 1.
Percentage of CD152 and CD154 T lymphocytes at postoperative days 3, 7, and 14 (%)
| Groups | CD152 | CD154 | ||||
|---|---|---|---|---|---|---|
| Day 3 | Day 7 | Day 14 | Day 3 | Day 7 | Day 14 | |
| Group A (n = 24) | 20.12 ± 0.71* | 30.71 ± 1.14* | 13.50 ± 0.71† | 10.00 ± 0.23† | 5.28 ± 0.69* | 14.67 ± 2.71* |
| Group B (n = 24) | 9.13 ± 0.40‡ | 7.90 ± 0.51‡ | 12.81 ± 0.92‡ | 9.91 ± 0.42‡ | 35.09 ± 1.01‡ | 20.22 ± 1.21‡ |
| Group C (n = 24) | 10.62 ± 0.35 | 8.62 ± 0.60 | 13.23 ± 0.81 | 11.01 ± 1.01 | 36.03 ± 1.39 | 22.78 ± 2.45 |
| F | 1240.03 | 1482.78 | 1.96 | 11.98 | 1722.16 | 43.76 |
| P | 0.00 | 0.00 | 0.17 | 0.00 | 0.00 | 0.00 |
Compared with Groups B and C, *P<0.05, †P>0.05. Compared with Group C, ‡P>0.05.
The levels of CD154 in Group A at postoperative days 7 and 14 were obviously lower than those in Groups B and C, while no significant difference could be found among values in the three groups at postoperative day 3. In addition, the levels of CD154 in Group A showed a downward trend from the 3rd to 7th day, with an upward trend from the 7th to 14th day (10.00 ± 0.23%, 5.28 ± 0.69%, and 14.67 ± 2.71% at postoperative days 3, 7, and 14, respectively, P < 0.05). No significant difference could be found between Groups B and C [Table 1].
Surface antigens in BV-2 cells
Levels of CD45 and CD54
The levels of both CD45 and CD54 in Group A at postoperative day 7 were obviously lower than those in Groups B and C, while no significant difference could be found among values in the three groups at postoperative days 3 and 14. In addition, the levels of both CD45 and CD54 in Group A showed a downward trend from the 3rd to 7th day, with an upward trend from the 7th to 14th day (CD45: 83.39 ± 2.56%, 82.74 ± 2.09%, and 87.56 ± 2.11%; CD54: 3.80 ± 0.24%, 0.94 ± 0.40%, and 3.41 ± 0.33% at postoperative days 3, 7, and 14, respectively, P < 0.05). No significant difference could be found between Groups B and C [Table 2 and Figure 5].
Table 2.
Percentage of CD45 and CD54 BV-2 cells at postoperative days 3, 7, and 14 (%)
| Groups | CD45 | CD54 | ||||
|---|---|---|---|---|---|---|
| Day 3 | Day 7 | Day 14 | Day 3 | Day 7 | Day 14 | |
| Group A (n = 24) | 83.39 ± 2.56* | 82.74 ± 2.09† | 87.56 ± 2.11* | 3.80 ± 0.24* | 0.94 ± 0.40† | 3.41 ± 0.33* |
| Group B (n = 24) | 86.31 ± 3.50‡ | 90.10 ± 0.91‡ | 89.01 ± 2.93‡ | 4.52 ± 0.40‡ | 9.29 ± 1.32‡ | 4.40 ± 0.42‡ |
| Group C (n = 24) | 84.62 ± 2.41 | 89.67 ± 1.02 | 87.45 ± 2.48 | 3.57 ± 0.12 | 10.01 ± 1.68 | 4.62 ± 0.50 |
| F | 2.38 | 57.18 | 1.22 | 20.02 | 108.79 | 18.93 |
| P | 0.12 | 0.00 | 0.32 | 0.00 | 0.00 | 0.00 |
Compared with Groups B and C, *P>0.05, †P<0.05. Compared with Group C, ‡P>0.05.
Figure 5.
Activity of BV-2 cells after co-culture with T-cells in Groups A–C. (a) CD45+; (b) CD54+ (The vertical axis indicate the number of cells, the red line in horizontal axis indicate the CD45+ and CD54+ cells, and the blue line in horizontal axis indicate the isotype control cells). Group A: Intrathymic injection of 100 μl myelin basic protein (1 mg/ml); Group B: Intrathymic injection of 100 μl phosphate-buffered saline (PBS); Group C: Sham operation group.
Expression of pro-inflammatory factors in BV-2 cells
The expressions of TNF-α, IL-1β, and iNOS in Group A were significantly lower than those in Groups B and C, and the values at postoperative day 7 were the lowest compared with those at postoperative days 3 and 14 (P < 0.05). No significant difference was found between Groups B and C [Figure 6].
Figure 6.
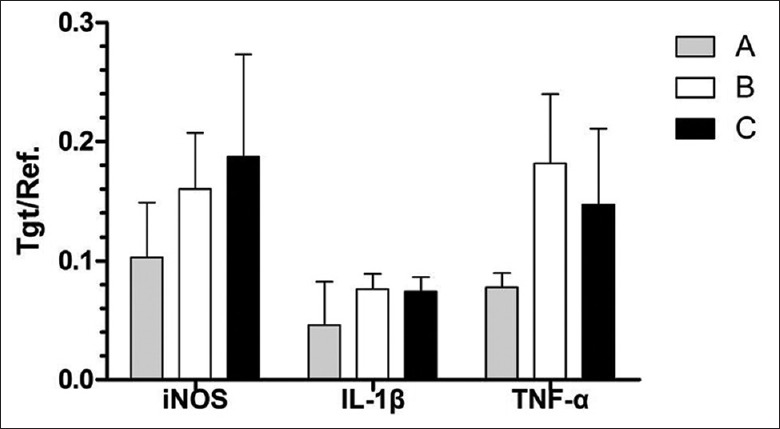
Expressions of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and inducible nitric oxide (iNOS) synthase in Group A were significantly lower than those in Groups B and C, and the values at postoperative day 7 were the lowest compared with those at postoperative days 3 and 14 (P < 0.05). No significant difference was found between Groups B and C. Group A: Intrathymic injection of 100 μl myelin basic protein (1 mg/ml); Group B: Intrathymic injection of 100 μl phosphate-buffered saline (PBS); Group C: Sham operation group.
DISCUSSION
Brain tissue contains a large amount of autoantigens, such as NSE, S-100β, and MBP. Once the BBB is broken, these autoantigens would get into the blood. Based on this theory, some researchers[15,16,17,18,19] implied these antigens as serum markers to evaluate the severity and prognosis of traumatic brain injuries (TBIs).
TBI is a major cause of neurological disability around the world, especially among the youths. The social and economic costs and harms including paralysis, speech disorder, and epilepsy should be paid more attention in the medical community. Recently, the researchers have targeted the secondary brain injury due to the primary brain injury. New viewpoints on the pathogenesis of secondary brain injury have been gradually recognized. Bellander et al.[20] demonstrated that genetic factors can regulate the neurodegeneration in the animal model of TBI. Teasdale et al.[21] reported the brain injured patients who carried apolipoprotein E (ApoEε4) suffered a worse clinical outcome than those without ApoEε4. Both of the studies revealed that genetic factors can determine the prognosis of TBI. Meanwhile, emerging data demonstrated that T lymphocytes, microglia, B-cells,[22] and even natural killer cells[23] play pivotal roles in the process of brain injury. Once BBB is damaged, brain antigens exposed in peripheral circulation would sensitize T lymphocytes, the sensitized T-cells then cross the BBB actively and accumulate around the lesion areas. However, the existence of T-cells cannot activate immune reaction effectively at this moment, another condition needed is that microglia converting from relatively static into active status. The performances of activated microglia include: (1) the alteration of cell morphology from the dendritic appearance to ameboid;[24] (2) The increased expression of surface antigens such as CD14, CD36, CD39, CD45, CD47, and CD54;[25,26] and (3) The accompanied up-regulation of IL-1β, TNF-α, and iNOS. Autoimmune MBP-specific T-cell lines express the CD4+ CD8− membrane phenotype, and their antigen-specific responses are restricted major histocompatibility complex-II (MHC-II). Only in this conditions can the activated BV-2 cells and sensitized T-cells form the immune complex to trigger the inflammatory response. However, the inflammatory response not only has the function of wound debridement and repair, but also can exacerbate the injury.[27] What we need is a way that could avoid disadvantages and minimize the inflammation caused by secondary damage at the same time.
As stated in the introduction, the clinical symptoms of the relapsing experimental autoimmune encephalomyelitis can be reduced by oral administration of MBP.[10] Immune tolerance through instilling self-antigen to nasal mucosa has neuroprotective effect for brain injury.[11] Nevertheless, their defects could be easily exposed: (1) oral tolerance needs a hundred-fold of antigen amount what nasal mucosa needs; (2) it is difficult to control the absorption rate of oral and/or nasal mucosa instillation of antigen; (3) the amount of clinical autologous brain antigen is limited; and (4) for patients with craniocerebral trauma combined with nasal facial injury, nasogastric tube indwelling, fasting, or water deprivation, the methods through oral and/or nasal mucosa administration of antigen are restricted. By contrast, the method of inducing thymus immune tolerance[28] could eliminate such defects. Gottrand et al.[13] found that the intrathymic injection of nonself-antigen could significantly weaken the immune reaction. Therefore, we hypothesized that could intrathymic injection of MBP induce immune tolerance so as to suppress autoimmune response?
As well known, T lymphocytes converge in the thymus for further mature process, some of these immature T-cells those can identify autoantigens are cleared out, while others continue the mature process and become T-cells those would not react to these autoantigens, and then enter the peripheral system along with blood circulation to participate in the immune response and immune regulation process. As the largest immune organ, spleen takes up about 25% lymphocytes of systemic circulation T-cells. Those lymphocytes directly participate in cellular immunity and adjust the distribution of peripheral blood T-cell. Therefore, we chose spleen T lymphocytes in this study. The purity of T-cells derived from the spleens could reach 85.0 ± 5.0% that can satisfy the experimental request. Kojima et al.[29] demonstrated that autoaggressive MBP-specific T-cell lines express the CD4+ CD8− membrane phenotype and their antigen-specific responses are MHC Class II restricted. The combination of IFN-γ and GM-CSF in the culture medium can up-regulate MHC Class II and co-stimulatory factor CD40 and B7 molecules. The ratio of CD4+/CD8+ T-cells indicated the strength of inflammation. CD152 is mainly expressed in activated CD4+ and CD8+ T-cells. Nonetheless, the affinity of the former two is significantly higher than the affinity of CD28 and B7. The integration of CD152 and B7 molecules can generate inhibitory signals to down-regulate or terminate the activation of T-cells. While the combination of CD28 and B7 would do the contrary.[30,31]
In this study, the performance of surface antigens and pro-inflammatory factors of T-cells and BV-2 cells in MBP group demonstrated that intrathymic injection of MBP could suppress the inflammatory response between the two co-cultured cells, indicating that intrathymic injection of MBP could induce immune tolerance. Nowadays, surgical brain injuries caused by cutting, electric coagulation, suction, and traction attract the researchers’ attention.[32,33] Intracranial tumors, especially glioma often require an elective operation. The operation can cause the secondary attack to the brain tissue. Although technologies of endoscope, functional mapping, and stereotactic localization applied in minimally, invasive surgery reduce the lesion area, auto-inflammation process triggered by the exposure of autoantigens is inevitable. For elective surgery, preoperatively intrathymic injection of MBP can induce the immune tolerance to alleviate auto-inflammation.
Due to the “wound healing” period as 3–7 days postinjuries,[34] and a “V”-shape trend that the CD4+/CD8+ T ratio in tolerance group showed, we speculate that the 7th day is an important period in which the inflammatory response is significantly inhibited. Meanwhile, this time point is the key period of wound repair, suggesting that short duration of immune tolerance is good for patients. With the development of minimally invasive operation and B-ultrasound technology, intrathymic injection without exposing the thorax will be increasingly simple, efficient, and safe. Intrathymic injection of MBP may become a promising treatment for TBI.
Study limitations
By studying the inflammatory response in vitro experimental, we proved that injection of MBP could down-regulate the inflammation response in the central nervous system. Even though a further study in vivo is needed to better investigate the immune tolerance induced between microglia cells and activated T lymphocytes.
Financial support and sponsorship
This study was supported by grants from Tianjin Municipal Science and Technology Commission (No. 13JCYBJC21100, No. 08ZCGYSF01600, and No. 13ZCZDSY01600).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Becker KJ, McCarron RM, Ruetzler C, Laban O, Sternberg E, Flanders KC, et al. Immunologic tolerance to myelin basic protein decreases stroke size after transient focal cerebral ischemia. Proc Natl Acad Sci U S A. 1997;94:10873–8. doi: 10.1073/pnas.94.20.10873. doi: 10.1073/pnas.94.20.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17:6–10. doi: 10.1016/j.jocn.2009.05.006. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romo-González T, Chavarría A, Pérez-H J. Central nervous system: A modified immune surveillance circuit? Brain Behav Immun. 2012;26:823–9. doi: 10.1016/j.bbi.2012.01.016. doi: 10.1016/j.bbi.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Sie C, Korn T, Mitsdoerffer M. Th17 cells in central nervous system autoimmunity. Exp Neurol. 2014;262(Pt A):18–27. doi: 10.1016/j.expneurol.2014.03.009. doi: 10.1155/2013/986789. [DOI] [PubMed] [Google Scholar]
- 5.Chabot S, Yong FP, Le DM, Metz LM, Myles T, Yong VW. Cytokine production in T lymphocyte-microglia interaction is attenuated by glatiramer acetate: A mechanism for therapeutic efficacy in multiple sclerosis. Mult Scler. 2002;8:299–306. doi: 10.1191/1352458502ms810oa. doi: 10.1191/1352458502ms810oa. [DOI] [PubMed] [Google Scholar]
- 6.Janke AD, Yong VW. Impact of IVIg on the interaction between activated T cells and microglia. Neurol Res. 2006;28:270–4. doi: 10.1179/016164106X98143. doi: 10.1179/016164106X98143. [DOI] [PubMed] [Google Scholar]
- 7.Zheng LT, Hwang J, Ock J, Lee MG, Lee WH, Suk K. The antipsychotic spiperone attenuates inflammatory response in cultured microglia via the reduction of proinflammatory cytokine expression and nitric oxide production. J Neurochem. 2008;107:1225–35. doi: 10.1111/j.1471-4159.2008.05675.x. doi: 10.1111/j.1471-4159.2008.05675.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Cha S, Lee MS, Cho GJ, Choi WS, Suk K. Role of antiproliferative B cell translocation gene-1 as an apoptotic sensitizer in activation-induced cell death of brain microglia. J Immunol. 2003;171:5802–11. doi: 10.4049/jimmunol.171.11.5802. doi: 10.4049/jimmunol.171.11.5802. [DOI] [PubMed] [Google Scholar]
- 9.Benson JM, Stuckman SS, Cox KL, Wardrop RM, Gienapp IE, Cross AH, et al. Oral administration of myelin basic protein is superior to myelin in suppressing established relapsing experimental autoimmune encephalomyelitis. J Immunol. 1999;162:6247–54. [PubMed] [Google Scholar]
- 10.Meyer AL, Benson JM, Gienapp IE, Cox KL, Whitacre CC. Suppression of murine chronic relapsing experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. J Immunol. 1996;157:4230–8. [PubMed] [Google Scholar]
- 11.Ayer RE, Jafarian N, Chen W, Applegate RL, Colohan AR, Zhang JH. Preoperative mucosal tolerance to brain antigens and a neuroprotective immune response following surgical brain injury: Laboratory investigation. J Neurosurg. 2012;116:246–53. doi: 10.3171/2011.8.JNS11883. doi: 10.3171/2011.8.JNS11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y, Kang J, Liu B, Fan W, Wu Q, Luo K, et al. An experimental study on thymus immune tolerance to treat surgical brain injury. Chin Med J. 2014;127:685–90. doi: 10.3760/cma.j.issn.0366-6999.20132851. [PubMed] [Google Scholar]
- 13.Gottrand G, Taleb K, Ragon I, Bergot AS, Goldstein JD, Marodon G. Intrathymic injection of lentiviral vector curtails the immune response in the periphery of normal mice. J Gene Med. 2012;14:90–9. doi: 10.1002/jgm.1650. doi: 10.1002/jgm.1650. [DOI] [PubMed] [Google Scholar]
- 14.Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–51. doi: 10.1016/j.bbi.2010.01.014. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Raabe A, Grolms C, Seifert V. Serum markers of brain damage and outcome prediction in patients after severe head injury. Br J Neurosurg. 1999;13:56–9. doi: 10.1080/02688699944195. [DOI] [PubMed] [Google Scholar]
- 16.Raabe A, Grolms C, Keller M, Döhnert J, Sorge O, Seifert V. Correlation of computed tomography findings and serum brain damage markers following severe head injury. Acta Neurochir (Wien) 1998;140:787–91. doi: 10.1007/s007010050180. [DOI] [PubMed] [Google Scholar]
- 17.Yan EB, Satgunaseelan L, Paul E, Bye N, Nguyen P, Agyapomaa D, et al. Post-traumatic hypoxia is associated with prolonged cerebral cytokine production, higher serum biomarker levels, and poor outcome in patients with severe traumatic brain injury. J Neurotrauma. 2014;31:618–29. doi: 10.1089/neu.2013.3087. doi: 10.1089/neu.2013.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beers SR, Berger RP, Adelson PD. Neurocognitive outcome and serum biomarkers in inflicted versus non-inflicted traumatic brain injury in young children. J Neurotrauma. 2007;24:97–105. doi: 10.1089/neu.2006.0055. doi: 10.1089/neu.2006.0055. [DOI] [PubMed] [Google Scholar]
- 19.Hergenroeder GW, Redell JB, Moore AN, Dash PK. Biomarkers in the clinical diagnosis and management of traumatic brain injury. Mol Diagn Ther. 2008;12:345–58. doi: 10.1007/BF03256301. doi: 10.2165/1250444-200812060-00002. [DOI] [PubMed] [Google Scholar]
- 20.Bellander BM, Lidman O, Ohlsson M, Meijer B, Piehl F, Svensson M. Genetic regulation of microglia activation, complement expression, and neurodegeneration in a rat model of traumatic brain injury. Exp Brain Res. 2010;205:103–14. doi: 10.1007/s00221-010-2342-z. doi: 10.1007/s00221-010-2342-z. [DOI] [PubMed] [Google Scholar]
- 21.Teasdale GM, Nicoll JA, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet. 1997;350:1069–71. doi: 10.1016/S0140-6736(97)04318-3. doi: 10.1016/S0140-6736(97)04318-3. [DOI] [PubMed] [Google Scholar]
- 22.Ankeny DP, Popovich PG. B cells and autoantibodies: Complex roles in CNS injury. Trends Immunol. 2010;31:332–8. doi: 10.1016/j.it.2010.06.006. doi: 10.1016/j.it.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gan Y, Liu Q, Wu W, Yin JX, Bai XF, Shen R, et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci U S A. 2014;111:2704–9. doi: 10.1073/pnas.1315943111. doi: 10.1073/pnas.1315943111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vo TS, Ngo DH, Ta QV, Wijesekara I, Kong CS, Kim SK. Protective effect of chitin oligosaccharides against lipopolysaccharide-induced inflammatory response in BV-2 microglia. Cell Immunol. 2012;277:14–21. doi: 10.1016/j.cellimm.2012.06.005. doi: 10.1016/j.cellimm.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Han D, Moon S, Kim Y, Min H, Kim Y. Characterization of the membrane proteome and N-glycoproteome in BV-2 mouse microglia by liquid chromatography-tandem mass spectrometry. BMC Genomics. 2014;15:95. doi: 10.1186/1471-2164-15-95. doi: 10.1186/1471-y2164-15-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein VM, Czub M, Schreiner N, Moore PF, Vandevelde M, Zurbriggen A, et al. Microglial cell activation in demyelinating canine distemper lesions. J Neuroimmunol. 2004;153:122–31. doi: 10.1016/j.jneuroim.2004.05.001. doi: 10.1016/j.jneuroim.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Brea D, Sobrino T, Ramos-Cabrer P, Castillo J. Inflammatory and neuroimmunomodulatory changes in acute cerebral ischemia. Cerebrovasc Dis. 2009;27(Suppl1):48–64. doi: 10.1159/000200441. doi: 10.1159/000200441. [DOI] [PubMed] [Google Scholar]
- 28.Naji A, Posselt AM, Barker CF. Pioneering work in immune tolerance. Science. 1992;258:727–8. doi: 10.1126/science.1439772. doi: 10.1126/science.1439772. [DOI] [PubMed] [Google Scholar]
- 29.Kojima K, Berger T, Lassmann H, Hinze-Selch D, Zhang Y, Gehrmann J, et al. Experimental autoimmune panencephalitis and uveoretinitis transferred to the Lewis rat by T lymphocytes specific for the S100 beta molecule, a calcium binding protein of astroglia. J Exp Med. 1994;180:817–29. doi: 10.1084/jem.180.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deppong CM, Bricker TL, Rannals BD, Van Rooijen N, Hsieh CS, Green JM. CTLA4Ig inhibits effector T cells through regulatory T cells and TGF-ß. J Immunol. 2013;191:3082–9. doi: 10.4049/jimmunol.1300830. doi: 10.4049/jimmunol.1300830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams K, Ulvestad E, Antel JP. B7/BB-1 antigen expression on adult human microglia studied in vitro and in situ. Eur J Immunol. 1994;24:3031–7. doi: 10.1002/eji.1830241217. doi: 10.1002/eji.1830241217. [DOI] [PubMed] [Google Scholar]
- 32.Jadhav V, Zhang JH. Surgical brain injury: Prevention is better than cure. Front Biosci. 2008;13:3793–7. doi: 10.2741/2968. doi: 10.2741/2968. [DOI] [PubMed] [Google Scholar]
- 33.Jadhav V, Solaroglu I, Obenaus A, Zhang JH. Neuroprotection against surgically induced brain injury. Surg Neurol. 2007;67:15–20. doi: 10.1016/j.surneu.2006.07.014. doi: 10.1016/j.surneu.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham TL, Cartagena CM, Lu XC, Konopko M, Dave JR, Tortella FC, et al. Correlations between blood-brain barrier disruption and neuroinflammation in an experimental model of penetrating ballistic-like brain injury. J Neurotrauma. 2014;31:505–14. doi: 10.1089/neu.2013.2965. doi: 10.1089/neu.2013.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]