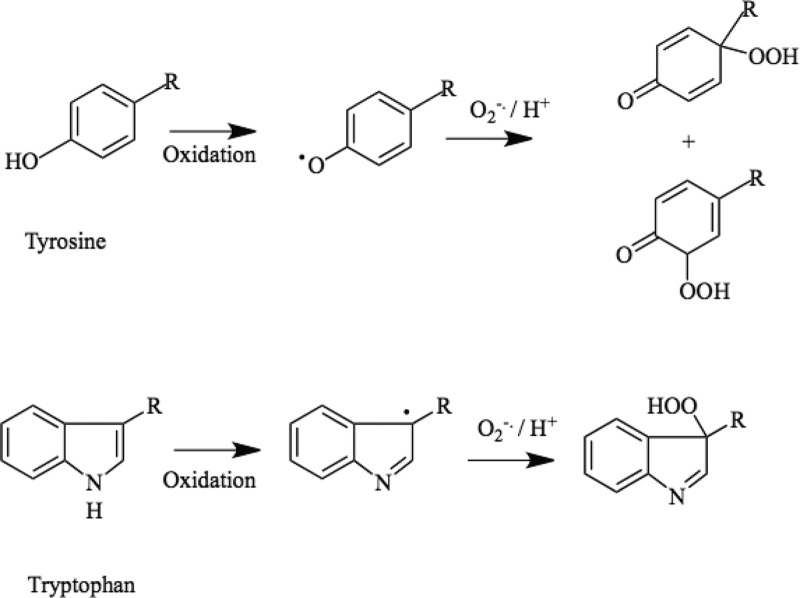
Figure 5. Formation of hydroperoxides on reaction of Tyr phenoxyl radicals and Trp indolyl radicals with the superoxide radical anion, O2−•.
In the case of the Tyr-derived species, these hydroperoxides can undergo further reactions with nucleophiles, including thiol, amine and amide groups to give more complex structures as a result of the presence of the conjugated double bond and carbonyl group, which is a reactive Michael acceptor. The resulting structures may retain the hydroperoxide function (see text).