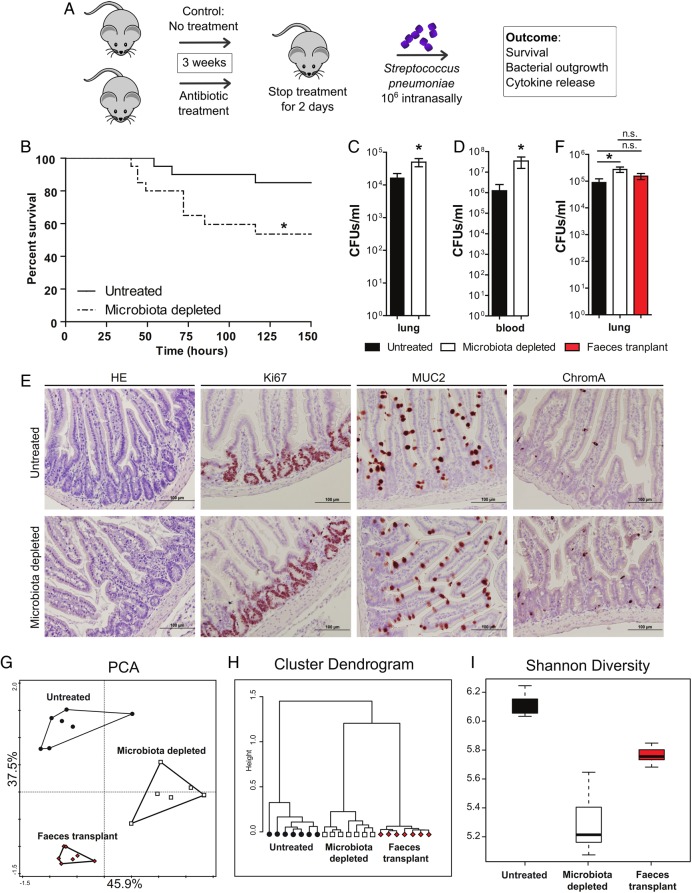
Figure 1.
Protective role of the gut microbiota during pneumococcal pneumonia. (A) Experimental design. Group of eight wild-type mice were treated for 3 weeks with broad-spectrum antibiotics (ampicillin, neomycin, metronidazole and vancomycin) in their drinking water compared with untreated controls. Two days post treatment mice received an intranasal challenge with 1×106 colony forming units (CFU) of Streptococcus pneumoniae. Subsequently, mouse survival, bacterial outgrowth and cytokine release were determined at various time points post S. pneumoniae infection. (B) Survival of mice treated with broad-spectrum antibiotics compared with untreated controls before intranasal challenge with 1×106 CFU of S. pneumoniae. (C) Pulmonary bacterial counts 6 h after S. pneumoniae infection in untreated (black) and microbiota-depleted (white) mice. (D) Blood bacterial counts 48 h after S. pneumoniae infection in untreated (black) and microbiota-depleted (white) mice. (E) Representative small intestine sections of untreated (upper row) and microbiota-depleted (lower row) mice demonstrate intact epithelial integrity with H&E, Ki67 (proliferation restricted in the crypt), MUC2 (goblet cell differentiation) and ChromograninA (neuroendocrine cell differentiation) stainings in both groups. (F) Effect of faecal microbiota transplantation to gut microbiota-depleted mice on lung bacterial counts 6 h after intranasal S. pneumoniae infection. (G) The magnitude by which the antibiotic protocol depleted the gut microbiota was assessed using a phylogenetic microarray in which microbiota composition samples were clustered based on principal component analysis (PCA), (H) Pearson Clustering and (I) the Shannon Diversity Index. Group size is 8–12 per group; results are shown as means±SEM; n.s. denotes not significant; *p<0.05.