ABSTRACT
The aim of this research was to study semantic abilities and their loss in mild cognitive impairment (MCI) and in dementia, while analyzing efficiency in the use of associative relations, within verbal and visuoperceptual modalities. Participants were split into 4 groups: 19 participants with amnestic MCI, 16 patients with mild Alzheimer disease (AD), 20 patients with moderate AD, and 20 healthy controls (HCs). All participants performed standardized neuropsychological tests and experimental (naming and semantic associations) tasks to evaluate verbal and visuoperceptual semantic abilities. We analyzed 4 associative relations (part/whole, function, superordinate, and contiguity) in both verbal and visuoperceptual code. Our results suggest a progressive impairment in semantic categorization knowledge, with worse performance in the AD groups relative to the MCI and HC groups. Our data show a different pattern in the 4 associative relations and the involvement of associative semantic relations already in the early stage of disease, as well as a different pattern of deterioration between verbal and visuoperceptual modalities. Our data indicate that the visuoperceptual semantic network appears to be less deteriorated than the verbal network in AD. The verbal semantic network may be more sensitive in detecting patients at an early stage of the disease.
KEYWORDS: associative relations, dementia, MCI, semantic ability, verbal and visuoperceptual access
Introduction
Concepts, the basic units of semantic memory, are considered by neuropsychological research as the essential elements of language and thinking (Olivetti Belardinelli, 2002).
Semantic knowledge appears to be structured in a hierarchical way. Concepts, one of the components of semantic knowledge, appear to be organized into multiple levels based upon their relationship with other concepts. These levels range from the superordinate (i.e., general category; e.g., animals), to the coordinate (i.e., a related subset of basic-level concepts belonging to the same superordinate category; e.g., dog and wolf), to the subordinate (i.e., a subset of the superordinate category; e.g., breed of dogs).
An interesting topic of research is focused on the manner in which semantic associative memory could deteriorate in the course of a progressive neurological disorder, such as dementia of the Alzheimer type. In fact, several studies have shown that a relevant deficit in semantic knowledge occurs in patients with Alzheimer disease (AD) in addition to memory, attention, and language impairment (Salmon & Bondi, 2009).
In contrast, this issue has not been explored sufficiently in the early stage of the disease. Although some evidence has been reported (Monsch et al., 1992; Rosser & Hodges, 1994), only few studies have sought to explain whether the deficit in the semantic associative network could be a prodromal symptom of dementia. A recent study (Caputi, Di Giacomo, Fiorenzi, & Passafiume, 2014), in which patients with a diagnosis of amnestic mild cognitive impairment (aMCI) were tested at the baseline and after 11 months, has suggested that deterioration of the semantic associative network occurs prior to deterioration rather than in the naming of the concepts.
The nature of semantic impairment is a controversial issue in the literature. The main hypotheses suggest that a semantic deficit could be related to the ability to access and handle semantic knowledge (Bayles, Tomoeda, Kaszniak, & Trosset, 1991; Laatu, Portin, Revonsuo, Tuisku, & Rinne, 1997; Nebes, 1989; Nebes & Brady, 1995; Nebes & Halligan, 1996); alternately, semantic deficits could be caused by a loss of semantic information (Binetti et al., 1995; Chan, Butters, & Salmon, 1997; Chertkow et al., 1996; Hodges, Salmon, & Butters, 1992; Martin & Fedio, 1983; Salmon, 2000).
Studies investigating semantic priming have highlighted that patients with AD show a significant priming effect in the superordinate category and a significant priming effect, albeit significantly reduced compared with healthy control participants, in the coordinate category (Glosser, Friedman, Grugan, Lee, & Grossman, 1998; Rogers & Friedman, 2008).
The results obtained by the use of implicit tasks appear to lean more toward loss of information, suggesting a mild impairment of semantic knowledge, and they would be consistent with the involvement of the anterior temporal lobe at the early stage of the disease (Frings et al., 2011; Rogers, Hocking, Noppeney, Mechelli, Gorno, Tempini, Patterson, & Price, 2008).
In clinical practice, semantic deficits are generally detected by naming or semantic fluency tasks, which require conscious retrieval of semantic information (explicit tasks; Rogers & Friedman, 2008). Marques (2007) has suggested that conceptual relations should be studied not only through naming tasks, but also through tasks that consider the common features of objects (e.g., visual, auditory, encyclopedic, tactile, spatial, etc.). In fact, his results indicated that processing conceptual networks that share few common features is more demanding than processing those that share a greater number of features, such as concepts at the superordinate level.
Analysis of the associative network in semantic memory shows that semantic impairment in AD reflects the segregation of concepts. In patients with AD, associative relations based on links that require verbal support for meaningful association deteriorate faster than those relations that are based on perceptual and functional features (Passafiume, De Federicis, Carbone, & Di Giacomo, 2012). These patterns of deterioration mirror the acquisition of semantic associative networks in life-span development (Di Giacomo, De Federicis, Pistelli, et al., 2012).
The use of tasks that analyze the shared relationships between concepts should suggest that at an early stage, patients with AD show deficits in associative relations first and then in semantic knowledge. Semantic memory can be assessed with several tasks that assess different aspects using different material types (visual and auditory stimuli).
Butler and colleagues (Butler, Brambati, Miller, & Gorno-Tempini, 2009) evaluated the relationship between regional gray matter volume and performance on a pictorial and verbal semantic association task in patients affected by neurodegenerative disease. These results revealed that different neural correlates are involved in verbal and visuospatial semantic processing, such that the visuoperceptual process is related to the right inferomedial temporal lobe, whereas the verbal process is related to the left temporal lobe.
The goal of this study was to analyze to what extent semantic associative relations deteriorate in MCI and AD using two input modalities: verbal and visuoperceptual. We then analyzed the differences between the knowledge of the objects, assessed by a naming task, and the semantic associative network of the same objects, assessed by an associative task, both in the verbal and the visuoperceptual systems.
The hypothesis was that the verbal and visuoperceptual modalities would have different effectiveness in detecting semantic associative deficits and in detecting MCI and that they would be organized differently. Specifically, the hypothesis was that the visuoperceptual semantic modality would be more preserved than the verbal modality because the picture could give access to the objects supplying information about the visual structure.
Methods
Participants and data collection
The study was conducted with 75 participants aged 60 to 80 years old; all were native Italian speakers. The sample was divided into four groups: (a) 19 patients with a diagnosis of aMCI (M age = 71.0 years, SD = 6.6 years), (b) 16 patients affected by probable mild AD (M age = 72.0 years, SD = 5.5 years), (c) 20 patients with probable moderate AD (M age = 74.3 years, SD = 5.8 years), and (d) 20 healthy controls (HCs) matched with the other groups by age, sex, and educational level (M age = 68.3 years, SD = 3.3 years).
Diagnoses were made by neurologists independent from the study according to the clinical guidelines in force in the clinics (Petersen criteria, Petersen et al., 1999) in the case of MCI and according to the National Institute of Neurological Disorders and Stroke–Alzheimer’s Disease and Related Disorders criteria (McKhann et al., 2011) in the case of probable mild and moderate AD.
The neurologist determined severity based on clinical criteria. Both patients and HC participants were subjected to a neuropsychological evaluation, and they were divided into groups on the basis of Mini Mental State Examination (MMSE) scores (Folstein, Folstein, & McHugh, 1975) as follows: moderate AD = 15 to 19, mild AD = 20 to 23, aMCI = 24 to 26, and HC > 27.
Patients were recruited from the day hospital of the Italian Hospital Group of Guidonia (Rome, Italy) and from the Neurogenetic Regional Centre of Lamezia Terme (Catanzaro, Italy).
HC participants were recruited by a physician, who identified patients without any history of alcohol or drug abuse or neurological or psychiatric diseases.
Only patients with sufficient comprehension and with good compliance with the testing procedure were included in the study. Psychologists were blind to the research objectives when conducting the test sections; the administration time was approximately 90 min. Informed consent was obtained from all participants or their caregivers. The study was approved by an ethics committee.
Materials
Patients were subjected to a neuropsychological evaluation that included standardized and experimental tests.
The standardized battery consisted of the MMSE (Folstein et al., 1975), Rey Auditory Verbal Learning Test (Rey, 1958), Raven Progressive Matrix ‘47 (Raven, 1947), and Wepman’s Visual Recognition Test (Wepman, Morency, & Seidl, 1975).
This battery was chosen to assess the neuropsychological impairments in the aMCI and AD groups and to verify the differences among these groups in the neuropsychological battery. The neuropsychological evaluation was also used to verify that the HC participants did not have any neuropsychological impairment (see Table 1).
Table 1. Standardized neuropsychological battery.
| RAVLT IR |
RAVLT DR |
WVRT |
RAVEN |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| HC | 0.68 | 0.83 | 1.49 | 0.62 | 1.23 | 0.15 | 1.19 | 0.41 |
| MCI | −0.01 | 1.12 | −0.25 | 0.33 | 0.13 | 0.67 | 0.26 | 0.79 |
| Mild AD | 0.15 | 0.78 | −0.56 | 0.16 | −0.62 | 0.61 | −0.52 | 0.31 |
| Moderate AD | −0.80 | 0.57 | −0.81 | 0.09 | −0.86 | 0.63 | −1.02 | 0.37 |
Note. Mean (±SD) correct answers for neuropsychological evaluation. RAVLT = Rey Auditory Verbal Learning Test; IR = Immediate Recall; DR = Delayed Recall; WVRT = Wepman’s Visual Recognition Test; RAVEN = Raven Progressive Matrices; HC = healthy controls; MCI = mild cognitive impairment; AD = Alzheimer disease.
The experimental battery was composed of two naming tasks, one verbal and one visuoperceptual: the Semantic Association Task verbal form (SAT) and the Semantic Association Task visuoperceptual form (VSAT).
The SAT has been extensively described elsewhere (Di Giacomo, De Federicis, Pistelli, et al., 2012). It is composed of 180 items, balanced between living and nonliving items and distributed across four associative relations: superordinate, contiguity, part/whole, and function.
Participants are asked to pair an object with its use (function relationship), its class membership (superordinate relationship), its single part (part/whole relationship), and its complement (contiguity relationship).
Briefly, the SAT consists of a set of 40 color photographs of concrete and frequently used objects (20 living and 20 nonliving items) repeated for each of the associative relations examined, as mentioned earlier. The function relation is composed of 20 nonliving items only, as appropriate specific functions are not universally recognized for living things (e.g., “cat” does not have a specific function).
The participant’s task was to choose, among three alternative words, the one that corresponded to the target presented at the top. The target was presented in two modalities, lexical and visuoperceptual, to allow for double access to the information. Two of the three word choices, presented below the target, were semantically unrelated to the stimulus and were similar to the target word in terms of length, frequency of use, and concreteness (see Figure 1).
Figure 1.

Example of an item on the Semantic Association Task (verbal form). Stimuli shown are words. The target is shown in two modalities (word and photo) to allow for double access to the information. In this example, participants had to pair the target (e.g., crocodile) with his class membership (e.g., reptiles).
The SAT also included the administration of a naming task, composed of 40 color pictures (gathered from the SAT) to verify knowledge of the object names. In the naming task, the participant’s task was to say the name of the object aloud.
The VSAT (Di Giacomo & Passafiume, 2014) is an experimental task composed of 40 color drawings (see Figure 2). As with the SAT, the items are balanced between living and nonliving items and are distributed across the four associative relations as described earlier.
Figure 2.
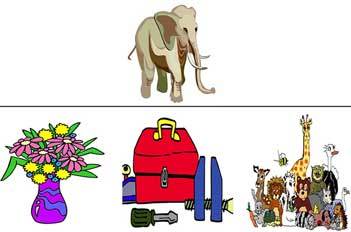
Example of an item on the Semantic Association Task (visuoperceptual form). Stimuli shown are colored drawings. In this example, participants had to pair the target (e.g., elephant) with his class membership (e.g., animals).
The participant’s task was to choose, among three alternative drawings, the one that corresponded to the target presented at the top.
The VSAT included the administration of a naming task, composed of 40 color drawings (gathered from the VSAT) to verify the knowledge of the object names.
In both the SAT and VSAT, there is no self-generation of the name of the object: The examiner pronounces aloud the name of the pictures and the words in the SAT and the name of the drawings in the VSAT; the participants have to say (or point to) the word (in the SAT) or the drawings (in the VSAT) that are semantically associated with the target.
For both the SAT and VSAT, a pilot study was conducted among healthy older adults to identify the types of stimuli that allowed for more efficient and rapid processing. We found that drawings ensured more effective processing than photographs in the visuoperceptual task (VSAT); on the contrary, in the verbal task (SAT), the image served to ensure that participants have understood the target word, so photos allowed the patient to be faster in stimulus identification.
Results
Statistical analysis
The data were evaluated by using Statistica 7 (Statsoft, 2006) for Windows. Analyses of variance (ANOVAs) were used to determine the degree of deterioration in the semantic associative relationship among the four groups, while taking into account the modalities of input (verbal and visuoperceptual) and output (naming and associative). Scores obtained in the experimental tasks were transformed to percentage of correct responses to compare the tasks that were composed of different numbers of items.
Results were considered statistically significant at the p < .05 level. A Tukey’s Honestly Significant Difference post-hoc test was used to determine the level of interaction.
Standard neuropsychological battery
Performance of the groups on the standardized tests was compared using a 4 (groups) × 4 (standardized tests) ANOVA for repeated measures.
The analysis revealed an interaction between groups and standardized tests, F(9, 213) = 5.57, p < .001, partial η2 = .191.
Post-hoc analysis for the interaction effect between groups and standardized tests showed that the HC group had significantly better performance than the aMCI, mild AD, and moderate AD groups (ps < .001).
There was, however, no difference between the standardized tests, F(3, 69) = 0.53, p > .05.
Input modalities: verbal and visuoperceptual modalities in associative tasks
Performance of the groups was compared using a 4 (groups) × 2 (modalities: verbal and visuoperceptual) ANOVA for repeated measures.
The results demonstrated a significant difference among groups, F(3, 71) = 52.78, p < .001, partial η2 = .690; a significant difference between modalities, F(1, 71) = 165.57, p < .001, partial η2 = .700; and a Groups × Modalities interaction, F(3, 71) = 11.30, p < .001, partial η2 = .323.
Post-hoc analysis revealed that performance on the VSAT was significantly greater than performance on the SAT in the pathological groups (p’s < .001; see Figure 3), but not for the HC group. In addition, in the visuoperceptual modality, the moderate AD group performed worse than the HC (p < .000) and aMCI (p < .001) groups; the mild AD group performed worse only than the HC group (p = .013). In the verbal modality, the MCI group performed worse than the HC group (p < .001) and better than the mild AD (p = .024) and moderate AD (p < .001) groups.
Figure 3.
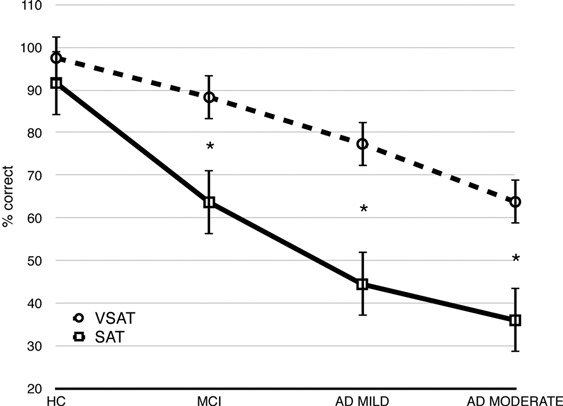
Mean (±SD) correct answers for the Semantic Association Task visuoperceptual (VSAT) and verbal (SAT) modalities. Differences were significant in mild cognitive impairment (MCI), mild Alzheimer disease (AD MILD), and moderate Alzheimer disease (AD MODERATE. ∗ ps < .001. HC = healthy controls.
To determine whether the interaction between groups and modalities was due to the confounding influence of the MMSE score, we conducted an analysis of covariance with the MMSE as a covariate. The analysis showed again the interaction effect of Groups × Modalities, F(3, 70) = 7.01, p < .001, partial η2 = .231.
To determine whether the interaction between groups and modalities was due to the confounding influence of age, we conducted an analysis of covariance with age as the covariate. The analysis also showed in this case the interaction effect of Groups × Modalities, F(3, 70) = 3.93, p < .05, partial η2 = .144.
Naming and associative tasks in the visuoperceptual modality
Performance of the groups on the VSAT was compared using a 4 (groups) × 2 (tasks: naming and associative) ANOVA for repeated measures.
The results demonstrated a significant main effect of group, F(3, 71) = 28.57, p < .001, partial η2 = .547. Post-hoc analysis showed that in visuoperceptual tasks, the HC (p < .001), MCI (p < .001), and mild AD (p = .012) groups performed better than patients with moderate AD; performance of the mild AD group was also worse than that of the HC (p < .001) and MCI (p = .035) groups. There was, however, no difference between naming and associative tasks, F(3, 71) = 1.02, p > .05.
Naming and associative tasks in the verbal modality
Performance of the groups was compared using a 4 (groups) × 2 (tasks: naming and associative) ANOVA for repeated measures.
The ANOVA demonstrated a main effect of group, F(3, 71) = 46.26, p < .001, partial η2 = .662, a main effect of task, F(1, 71) = 207.60, p < .001, partial η2 = .745, and an interaction between group and modality, F(3, 71) = 14.36, p < .001, partial η2 = .378.
Post-hoc analysis revealed that the groups were all significantly different from one another, with the HC group outperforming the remaining three groups (p < .001); the MCI group had significantly better performance than the mild AD (p = .008) and the moderate AD groups (p < .001). In addition, the moderate AD group performed worse than the mild AD group (p = .029).
In verbal access (see Figure 4), performance on the associative task was worse than in the naming task in the MCI (p < .001), mild AD (p < .001), and moderate AD (p < .001) groups but not in the HC group (p = .480). The HC and MCI groups outperformed the mild AD and moderate AD groups (p < .001) on the SAT. In addition, the HC group performed better than the MCI group on the SAT (p < .001) and better than the moderate AD group on the naming task (p < .001).
Figure 4.
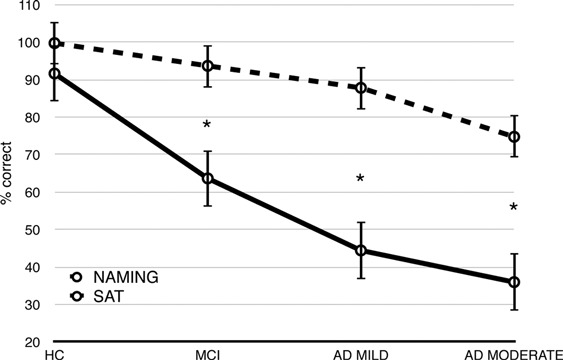
Mean (±SD) correct answers for the naming task and Semantic Association Task (SAT) in verbal modality. Differences were significant for mild cognitive impairment (MCI), mild Alzheimer disease (AD MILD), and moderate Alzheimer disease (AD MODERATE. ∗ p’s < .001. HC = healthy controls.
To determine whether the interaction between groups and modalities was due to the confounding influence of the MMSE score, we conducted an analysis of covariance with the MMSE as the covariate. The analysis showed again the interaction effect of Groups × Modalities, F(3, 70) = 7.88, p < .001, partial η2 = .253.
Associative relationships: function, part/whole, contiguity, and superordinate on the VSAT
For the VSAT, performance of the groups on the associative relations task was compared using a 4 (groups) × 4 (relationship) ANOVA for repeated measures.
The ANOVA demonstrated a main effect of group, F(3, 71) = 32.62, p < .001, partial η2 = .580, a main effect of associative relationship, F(3, 213) = 9.30, p < .001, partial η2 = .116, and an interaction between group and associative relationship, F(9, 213) = 5.28, p < .001, partial η2 = .122.
To decompose the main effect of group, post-hoc analysis demonstrated that both the HC and MCI groups had significantly better performance than the mild AD and moderate AD groups (p < .05). In addition, the mild AD group outperformed the moderate AD group (p = .003).
Post-hoc analysis to decompose the main effect of associative relationship revealed that performance for the contiguity and superordinate categories was significantly better than performance for the function and part/whole categories (all p’s < .05).
With regard to the interaction between group and associative relationship (see Figure 5), post-hoc analysis revealed that the function category was significantly worse than the contiguity (p < .001) and the superordinate (p < .001) categories only in the moderate AD group; furthermore, performance for the function relationship in the moderate AD group was significantly worse than performance for the function relationship in the HC (p < .001) and MCI groups (p < .001). Performance for the other associative relationship in the moderate AD group was significantly worse only than that in the HC group (p < .05).
Figure 5.
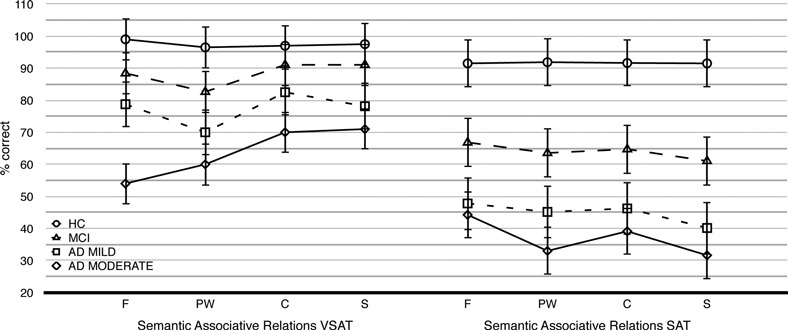
(A) Mean (±SD) correct answers for semantic associative relationships in the Semantic Association Task visuoperceptual (VSAT) modality. (B) Mean (±SE) correct answers for semantic associative relationships in the Semantic Association Task verbal (SAT) modality. Note. F = function relationship; PW = part/whole relationship; C = contiguity relationship; S = superordinate relationship.
To determine whether the interaction between groups and associative relationships was due to the confounding influence of the MMSE score, we conducted an analysis of covariance with the MMSE as a covariate. The analysis showed again the main effect of associative relationships, F(3, 68) = 3.56, p < .05, partial η2 = .136, and the interaction effect between group and associative relationships, F(9, 210) = 2.64, p < .01, partial η2 = .102.
Associative relations: function, part/whole, contiguity, and superordinate on the SAT
Performance of the groups on the associative relationship was compared using a 4 (groups) × 4 (relationship) ANOVA) for repeated measures.
The analysis revealed a main effect of group, F(3, 71) = 43.09, p < .001, partial η2 = .645, a main effect of associative relationship, F(3, 213) = 48.79, p < .001, partial η2 = .407, and an interaction between group and associative relationship, F(9, 213) = 10.93, p < .001, partial η2 = .316.
Post-hoc analysis for the main effect on group showed that both the HC and MCI groups had significantly better performance than the mild AD and moderate AD groups (p’s < .05). In addition, the MCI group performed worse than the HC group (p < .001).
Post-hoc analysis for the main effect of associative relationship revealed that the relationship types were all significantly different from one another. The strongest performance was for the function relationship (p’s < .001) relative to each of the other categories; the contiguity relationship was stronger than the part/whole and superordinate relationships (p’s < .001) but more deteriorated than the function relationship (p < .001). The weakest performance was for the superordinate relationship (p’s < .001) relative to each of the other relationships.
With regard to the Group × Associative Relations interaction effect (see Figure 5), post-hoc analysis revealed that within the MCI group, performance for the superordinate relationship was worse than performance for the function (p < .001) relationship. Within the mild AD group, the superordinate relationship was worse than each of the other associative relationships (i.e., function, part/whole, contiguity; p’s < .001).
In the moderate AD group, the superordinate relationship was poorer than the function (p < .001) and contiguity (p < .001) relationships but not the part/whole relationship. The part/whole relationship was significantly worse than both function (p < .001) and contiguity (p < .001).
Performance on each of the associative relationships in the mild and moderate AD groups was significantly worse than the HC group performance (all p’s < .05).
To determine whether the interaction between groups and associative relationships was due to the confounding influence of the MMSE score, we conducted an analysis of covariance with MMSE as the covariate. The analysis showed again the main effect of associative relationships, F(3, 68) = 13.54, p < .001, partial η2 = .374, and the interaction effect between group and associative relationships, F(9, 210) = 2.90, p < .005, partial η2 = .111.
Discussion and conclusion
In this study, we examined the deterioration of semantic associative relationships in MCI and AD, while considering two modalities of organization. We compared four groups of participants, including those with MCI, those with mild and moderate AD, and HC older adults to determine if it is possible to identify a prodromal symptom in the linkage of concepts (i.e., semantic associative relations) and if there is a difference between verbal and visuoperceptual modalities in detecting semantic associative impairment. The goal of the present study was also to understand if naming of concepts is more or less deteriorated in AD than the semantic links between concepts.
The findings suggested that verbal input was more efficient than visuoperceptual input to detect patients who were at the early stage of cognitive decline, such that our semantic task with verbal input distinguished patients with MCI from both the HC and AD groups. On the contrary, performance on our semantic task with visuoperceptual input was better than performance on the verbally mediated task, and the visuoperceptual task was unable to discern the groups.
Our results suggest that participants may gain access to the semantic associative network in two ways, one visuoperceptual and one verbal. It seems that the associative relationships elicited by visuoperceptual stimuli support the associative semantic ability when the breakdown of the verbally mediated semantic associative relationships occurs. This hypothesis is coherent with the idea that the deterioration of the semantic network is reverse to the acquisition of the semantic associative relationships in childhood (Di Giacomo, De Federicis, Pistelli, et al., 2012).
The different organization of the two systems is also apparent when comparing the two types of tasks (naming and associative tasks). In the visuoperceptual modality, the tasks had the same pattern of deterioration, regardless of whether the participants answered about the knowledge of the object or about the associative network. In the verbal modality, however, the associative network was more impaired than the naming of the objects. As such, the deficit observed in AD appeared to be derived from an inability to access explicit semantic information contained in the networks when the naming of the concept was still available.
This finding is in line with other previous findings (Di Giacomo, De Federicis, Pistelli, et al., 2012; Passafiume et al., 2012), which showed that the semantic impairment in AD starts with difficulty in using the semantic associative relationships and not with a deficit in naming of the concepts.
The different organization of semantic systems, visuoperceptual and verbal, is in line with previous literature on semantic priming (Ballesteros, Reales, & Mayas, 2007; Boccia, Silveri, & Guariglia, 2014), which used different types of stimuli (verbal and visuoperceptual) and different types of processing (identification and production).
Tasks that require stimulus identification (Ballesteros et al., 2007) rather than production (Boccia et al., 2014) show spared visuoperceptual priming; these differences are probably due to the different level of processing involved.
Moreover, Peraita and colleagues (Peraita, Diaz, & Anllo-Vento, 2008), who used an analogical reasoning task to investigate the semantic network in visuoperceptual modality and a sentence verification task in verbal modality, showed that functional relationships appeared to be less affected by AD and that part/whole relationships had the fastest decline across the disease in the verbal modality; instead, they found no differences among groups in handling the semantic associative relationship in the visuoperceptual tasks. They also found that taxonomic relationships were the most difficult in both tasks.
Our data showed, as did Peraita et al.’s (2008), a progressive impairment in using the semantic associative network. However, our findings are discrepant regarding which semantic associative relationship is impaired first. Consistent with Peraita et al., in the verbal modality, we found that the function relationship appeared to be less affected relative to each of the other relationships. However, we found that the superordinate relationship has the fastest decline across the disease, whereas Peraita et al. demonstrated a part/whole relationship.
In the visuoperceptual modality, we found differences among the semantic relationships but only at the most severe level of disease (in which the most difficult relationship is the function category compared with contiguity and superordinate relationships).
This discrepancy is probably due to the differences between our tasks, which required participants to associate a target to an item semantically related, and Peraita et al.’s (2008) task, which required an inference about the correctness of a sentence in the verbal task or an analogical reasoning in the visuoperceptual task.
In the moderate AD group, the associative network appeared to be damaged both in hierarchical and nonhierarchical classifications. It appears to be more difficult for these patients to compare two objects belonging to different categories (cross-classification) than objects belonging to the same category (classification) if the system involved is the visuoperceptual system as compared with the verbal system.
Our data show that in the verbal modality, patients have difficulty in establishing the class to which the objects belong and in determining the hierarchical organization. A possible explanation is that the verbal task requires participants to have access to the shared and distinctive features of the object and of the different class memberships. These requirements are different from the requirements of the visuoperceptual task, in which these features are represented in the stimuli.
A picture directly activates semantic features corresponding to properties of the objects present in the images: Pairing an object with its class membership when the distinctive features are visible is likely simpler than recalling these features without any suggestion.
In conclusion, our results suggest that the deterioration of semantic abilities in dementia of the Alzheimer type and in aMCI is different in visuoperceptual and verbal modalities and that a verbal associative task is more sensitive than a visuoperceptual associative task in detecting patients at an early stage of the disease.
Further research needs to identify more sensitive instruments to clarify the role of visuoperceptual semantic storage and its disruption in the early phases of disease.
In addition, our results suggest that the examination of semantic skills should be extended beyond tests that involve verbal production (e.g., verbal fluency and naming tasks) and should include measures that activate conceptual linkage, possibly using multiple access modalities.
Further research needs to clarify whether semantic association ability could be a strong diagnostic indicator for detecting semantic deficits in the earliest phases of disease and needs to compare its sensitivity relative to neuropsychological tests that are generally used for clinical assessment.
Though our results are in line with previous investigations, our conclusions are limited by the small number of participants included in our study. In addition, our study was cross-sectional; a longitudinal study is necessary to determine whether patients affected by MCI with deficits in the semantic associative network are at greater risk for conversion to AD.
In addition, we did not have any data about the presence of mood disorders in these patients; further research needs to clarify whether there is a correlation between mood disorders and semantic associative impairment in AD.
Future investigations may also be directed toward examining the benefit of using semantic associative tasks in basic neuropsychological assessment and in using visuoperceptual stimuli in the rehabilitation of the semantic network.
Acknowledgements
We thank the Research and Editing Consulting Program, especially Dr. Emily Briceno for the editing support. Preliminary results were presented at the 22nd meeting of the European Neurological Society in Prague, Czech Republic, in 2012 (Di Giacomo, De Federicis, Caputi, & Passafiume, 2012).
References
- Ballesteros S., Reales J. M., Mayas J. Picture priming in normal aging and Alzheimer’s disease. Psicothema. 2007:239–244. [PubMed] [Google Scholar]
- Bayles K. A., Tomoeda C. K., Kaszniak A. W., Trosset M. W. Alzheimer’s disease effects on semantic memory: Loss of structure or impaired processing? Journal of Cognitive Neuroscience. 1991:166–182. doi: 10.1162/jocn.1991.3.2.166. [DOI] [PubMed] [Google Scholar]
- Binetti G., Magni E., Cappa S. F., Padovani A., Bianchetti A., Trabucchi M. Semantic memory in Alzheimer’s disease: An analysis of category fluency. Journal of Clinical and Experimental Neuropsychology. 1995:82–89. doi: 10.1080/13803399508406584. [DOI] [PubMed] [Google Scholar]
- Boccia M., Silveri M. C., Guariglia C. Visuo-perceptive priming in Alzheimer’s disease: Evidence for a multi-componential implicit memory system. Journal of Alzheimer’s Disease. 2014:455–463. doi: 10.3233/JAD-131775. [DOI] [PubMed] [Google Scholar]
- Butler C. R., Brambati S. M., Miller B. L., Gorno-Tempini M.-L. The neural correlates of verbal and nonverbal semantic processing deficits in neurodegenerative disease. Cognitive and Behavioral Neurology. 2009;(2):73–80. doi: 10.1097/wnn.0b013e318197925d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputi N., Di Giacomo D., Fiorenzi D., Passafiume D. 2014. May-Jun. Semantic associative abilities in mild cognitive impairment: Gender asymmetries? Poster session presented at the 24th meeting of the European Neurological Society, Istanbul, Turkey. [Google Scholar]
- Chan A. S., Butters N., Salmon D. P. The deterioration of semantic networks in patients with Alzheimer’s disease: A cross sectional study. Neuropsychologia. 1997:241–248. doi: 10.1016/s0028-3932(96)00067-x. [DOI] [PubMed] [Google Scholar]
- Chertkow H., Bub D., Murtha S., Beauregard M., Gold D., Hosein C., Evans A. 1996. Variability of brain regions in word processing: Evidence for dissociation of processing levels. Paper presented at the third annual meeting of the Cognitive Neuroscience Society, San Francisco, CA. [Google Scholar]
- Di Giacomo D., De Federicis L. S., Caputi N., Passafiume D. Verbal and visuoperceptive semantic association in aMCI and AD patients. Journal of Neurology. 2012 Jun;(Suppl. 1):S170. [Google Scholar]
- Di Giacomo D., De Federicis L. S., Pistelli M., Fiorenzi D., Sodani E., Carbone G., Passafiume D. The loss of conceptual associations in mild Alzheimer’s dementia. Journal of Clinical and Experimental Neuropsychology. 2012:643–653. doi: 10.1080/13803395.2012.667393. [DOI] [PubMed] [Google Scholar]
- Di Giacomo D., Passafiume D. Semantic Association Task (SAT). manual. Milano, Italy: Franco Angeli; 2014. [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. ‘Mini-mental state’: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frings L., Kloppel S., Teipel S., Peters O., Frolich L., Pantel J., … . Hull M. Left anterior temporal lobe sustains naming in Alzheimer’s dementia and mild cognitive impairment. Current Alzheimer Research. 2011:893–901. doi: 10.2174/156720511798192673. [DOI] [PubMed] [Google Scholar]
- Glosser G., Friedman R., Grugan P. K., Lee J., Grossman M. Lexical semantic and associative priming in Alzheimer’s disease. Neuropsychology. 1998:218–224. doi: 10.1037//0894-4105.12.2.218. [DOI] [PubMed] [Google Scholar]
- Hodges J. R., Salmon D. P., Butters N. Semantic memory impairment in Alzheimer’s disease: Failure of access or degraded knowledge? Neuropsychologia. 1992:301–314. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Laatu S., Portin R., Revonsuo A., Tuisku S., Rinne J. Knowledge of concept meanings in Alzheimer’s disease. Cortex. 1997:27–45. doi: 10.1016/s0010-9452(97)80003-2. [DOI] [PubMed] [Google Scholar]
- Marques J. F. The general/specific breakdown of semantic memory and the nature of superordinate knowledge: Insights from superordinate and basic-level feature norms. Cognitive Neuropsychology. 2007:879–903. doi: 10.1080/02643290701789436. [DOI] [PubMed] [Google Scholar]
- Martin A., Fedio P. Word production and comprehension in Alzheimer’s disease: The breakdown of semantic knowledge. Brain Language. 1983:124–141. doi: 10.1016/0093-934x(83)90059-7. [DOI] [PubMed] [Google Scholar]
- McKhann G. M., Knopman D. S., Chertkow H., Hyman B. T., Jack C. R., Jr., Kawas C. H., … . Phelps C. H. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsch A. U., Bondi M. W., Butters N., Salmon D. P., Katzman R., Thal L. J. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Archives of Neurology. 1992:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Nebes R. D. Semantic memory in Alzheimer’s disease. Psychological Bulletin. 1989:377–394. doi: 10.1037//0033-2909.106.3.377. [DOI] [PubMed] [Google Scholar]
- Nebes R. D., Brady C. B. Preservation of ambiguous word meanings in patients with Alzheimer’s disease. Developmental Neuropsychology. 1995:253–267. [Google Scholar]
- Nebes R. D., Halligan E. M. Sentence context influences the interpretation of word meaning by Alzheimer patients. Brain and Language. 1996:233–245. doi: 10.1006/brln.1996.0073. [DOI] [PubMed] [Google Scholar]
- Olivetti Belardinelli M. Aggiornamento tematico su ‘Concetti e categorizzazione’ [Upgrade on ‘Concepts and Categorization’] In: Schönpflug W., editor; Schönpflug U., editor. Istituzioni di psicologia generale [Manual of psychology] Padua, Italy: CEDAM; 2002. pp. 228–231. [Google Scholar]
- Passafiume D., De Federicis L. S., Carbone G., Di Giacomo D. Loss of semantic associative categories in patients with Alzheimer’s disease. Applied Neuropsychology: Adult. 2012:305–311. doi: 10.1080/09084282.2012.670160. [DOI] [PubMed] [Google Scholar]
- Peraita H., Diaz C., Anllo-Vento L. Processing of semantic relations in normal aging and Alzheimer’s disease. Archives of Clinical Neuropsychology. 2008:33–46. doi: 10.1016/j.acn.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Petersen R. C., Smith G. E., Waring S. C., Ivnik R. J., Tangalos E. G., Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Raven J. C. Progressive matrices. London, UK: H. K. Lewis; 1947. [Google Scholar]
- Rey A. L’examen clinique en psychologie [Clinical assessment in psychology] Paris, France: Presses Universitaires de Paris; 1958. [Google Scholar]
- Rogers S. L., Friedman R. B. The underlying mechanisms of semantic memory loss in Alzheimer’s disease and semantic dementia. Neuropsychologia. 2008:12–21. doi: 10.1016/j.neuropsychologia.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. T., Hocking J., Noppeney U., Mechelli A., Gorno-Tempini M. L., Patterson K., Price C. J. Anterior temporal cortex and semantic memory: Reconciling findings from neuropsychology and functional imaging. Cognitive, Affective, & Behavioral Neuroscience. 2006:201–213. doi: 10.3758/cabn.6.3.201. [DOI] [PubMed] [Google Scholar]
- Rosser A., Hodges J. R. Initial letter and semantic category fluency in Alzheimer’s disease, Huntington’s disease, and progressive supranuclear palsy. Journal of Neurology, Neurosurgery & Psychiatry. 1994:1389–1394. doi: 10.1136/jnnp.57.11.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon D. P. Disorders of memory in Alzheimer’s disease. In: Boller F., editor; Grafman J., editor. Handbook of neuropsychology: Vol. 2. Memory and its disorders ( 2nd ed. Amsterdam, The Netherlands: Elsevier; 2000. pp. 155–195. [Google Scholar]
- Salmon D. P., Bondi M. W. Neuropsychological assessment of dementia. Annual Review of Psychology. 2009:257–282. doi: 10.1146/annurev.psych.57.102904.190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistica. Statsoft Italia s.r.l; 2006. Statsoft. [Google Scholar]
- Wepman J. M., Morency A., Seidl M. Visual Discrimination Test. Chicago, IL: Language Research Associates; 1975. [Google Scholar]


