Table 1.
Reaction screening data.
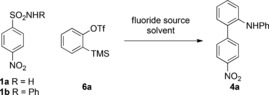
| Entry | R | 1/6 (equiv) | Fluoride (equiv) | Yield [%][a] |
|---|---|---|---|---|
| 1 | H | 2:1 | CsF (4) | 16[b,c] |
| 2 | H | 2:1 | KF + 18‐c‐6 (4) | 31[b] |
| 3 | H | 2:1 | TBAF (1) | 38[b] |
| 4 | H | 1:2 | KF + 18‐c‐6 (2) | 63 |
| 5 | Ph | 1:2 | KF + 18‐c‐6 (2) | 66 |
| 6 | Ph | 1:1 | KF + 18‐c‐6 (2) | 56 |
| 7 | Ph | 2:1 | KF + 18‐c‐6 (2) | 31 |
| 8 | Ph | 1:1 | KF + 18‐c‐6 (3) | 62[d] |
| 9 | Ph | 1:1 | KF + 18‐c‐6 (4) | 59 |
| 10 | Ph | 1:1 | CsF (3) | 45 |
| 11 | Ph | 1:1 | CsF (3) | 64[c] |
[a] Reaction conditions: Sulfonamide (1; 0.1 mmol), trimethylsilylphenyl triflate (6; 0.1 mmol), fluoride source, THF (0.1 m), reflux, 24 hours. Yield is that of the isolated 4 a. [b] Ambient temperature and 16 hours. [c] Acetonitrile used as solvent. [d] Increasing scale to 1 mmol afforded 4 a in a 56 % yield. Tf=trifluoromethanesulfonyl, TMS=trimethylsilyl.
