Abstract
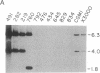
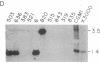
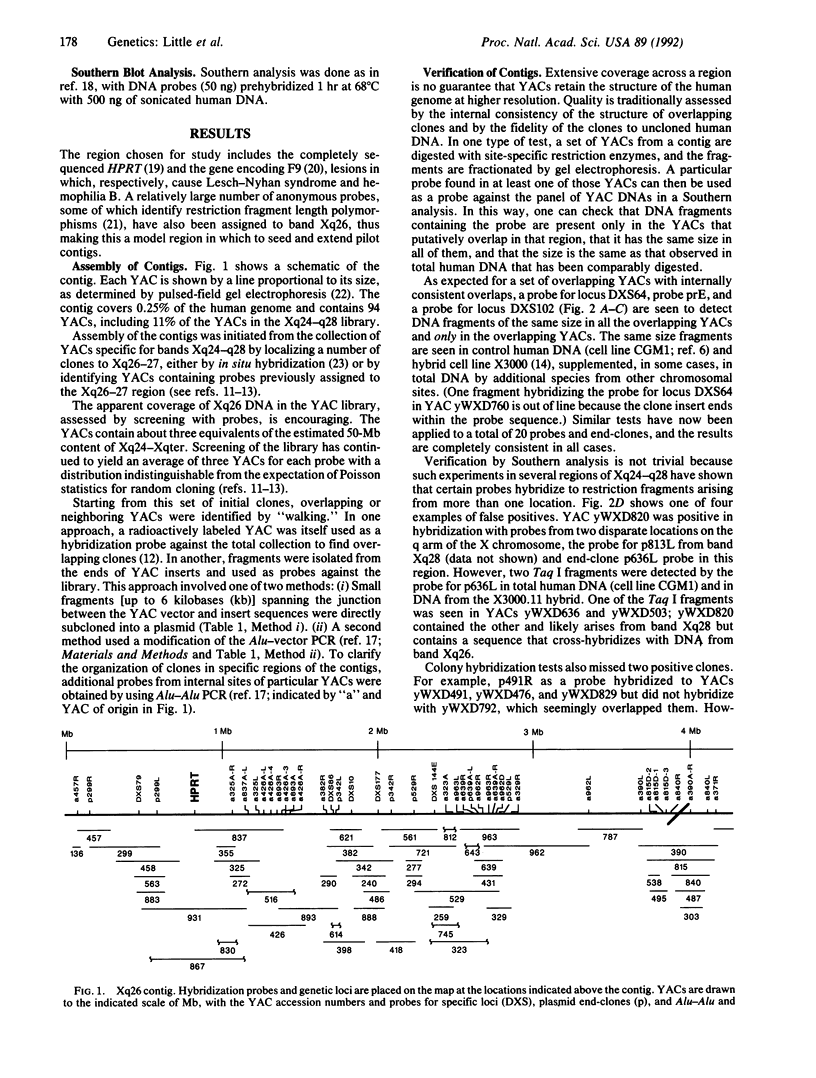
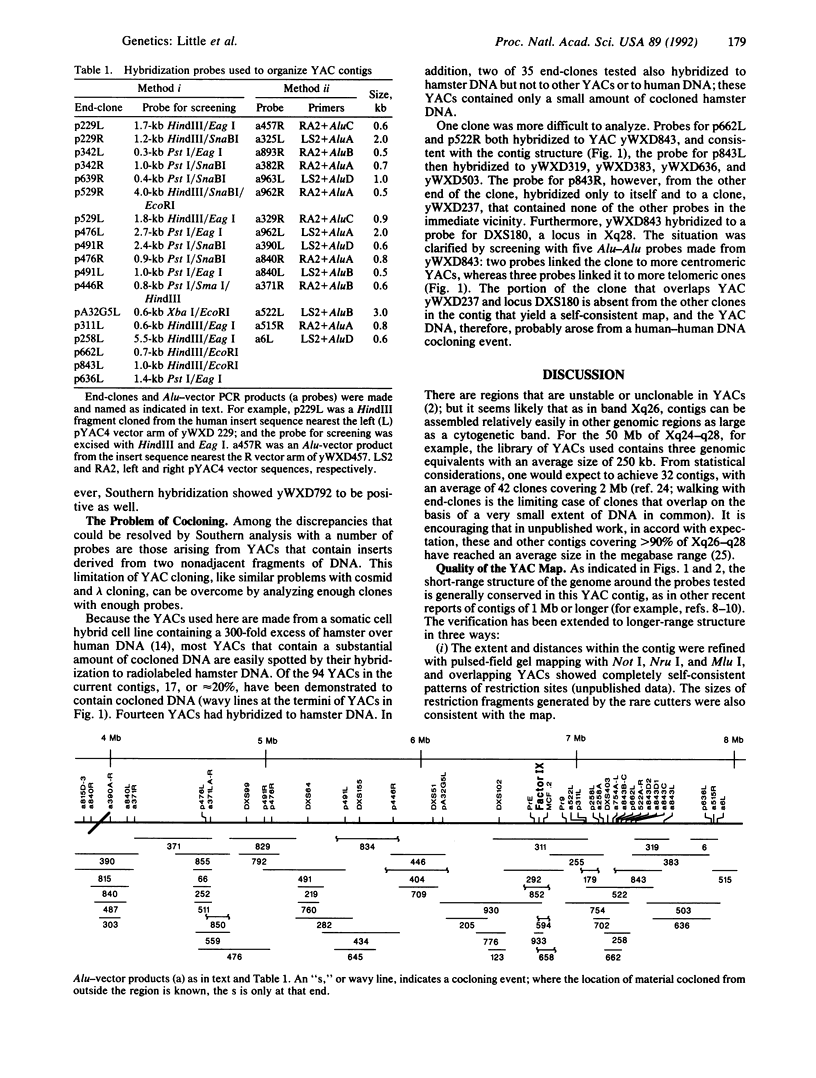
A successful test is reported to generate long-range contiguous coverage of DNA from a human cytogenetic band in overlapping yeast artificial chromosomes (YACs). Seed YACs in band Xq26 were recovered from a targeted library of clones from Xq24-q28 with 14 probes, including probes for the hypoxanthine guanine phosphoribosyltransferase- and coagulation factor IX-encoding genes and nine probes used in linkage mapping. Neighboring YACs were then identified by 25 "walking" steps with end-clones, and the content of 71 probes in cognate YACs was verified by further hybridization analyses. The resultant contig extends across 8 million base pairs, including most of band Xq26, with an order of markers consistent with linkage data. YAC-based mapping, thus, permits steps toward a fully integrated physical and genetic map and is probably adequate to sustain most of the human genome project.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abidi F. E., Wada M., Little R. D., Schlessinger D. Yeast artificial chromosomes containing human Xq24-Xq28 DNA: library construction and representation of probe sequences. Genomics. 1990 Jul;7(3):363–376. doi: 10.1016/0888-7543(90)90170-y. [DOI] [PubMed] [Google Scholar]
- Anson D. S., Blake D. J., Winship P. R., Birnbaum D., Brownlee G. G. Nullisomic deletion of the mcf.2 transforming gene in two haemophilia B patients. EMBO J. 1988 Sep;7(9):2795–2799. doi: 10.1002/j.1460-2075.1988.tb03134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetta L., Kuehn S. E., Huang A., Law D. J., Kalikin L. M., Koi M., Reeve A. E., Brownstein B. H., Yeger H., Williams B. R. Wilms tumor locus on 11p13 defined by multiple CpG island-associated transcripts. Science. 1990 Nov 16;250(4983):994–997. doi: 10.1126/science.2173146. [DOI] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G. The molecular pathology of haemophilia B. Fourth Wellcome Trust lecture. Biochem Soc Trans. 1987 Feb;15(1):1–8. doi: 10.1042/bst0150001. [DOI] [PubMed] [Google Scholar]
- Brownstein B. H., Silverman G. A., Little R. D., Burke D. T., Korsmeyer S. J., Schlessinger D., Olson M. V. Isolation of single-copy human genes from a library of yeast artificial chromosome clones. Science. 1989 Jun 16;244(4910):1348–1351. doi: 10.1126/science.2544027. [DOI] [PubMed] [Google Scholar]
- Burke D. T., Carle G. F., Olson M. V. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science. 1987 May 15;236(4803):806–812. doi: 10.1126/science.3033825. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Coulson A., Waterston R., Kiff J., Sulston J., Kohara Y. Genome linking with yeast artificial chromosomes. Nature. 1988 Sep 8;335(6186):184–186. doi: 10.1038/335184a0. [DOI] [PubMed] [Google Scholar]
- Davies K. E., Mandel J. L., Monaco A. P., Nussbaum R. L., Willard H. F. Report of the committee on the genetic constitution of the X chromosome. Cytogenet Cell Genet. 1990;55(1-4):254–313. doi: 10.1159/000133019. [DOI] [PubMed] [Google Scholar]
- Edwards A., Voss H., Rice P., Civitello A., Stegemann J., Schwager C., Zimmermann J., Erfle H., Caskey C. T., Ansorge W. Automated DNA sequencing of the human HPRT locus. Genomics. 1990 Apr;6(4):593–608. doi: 10.1016/0888-7543(90)90493-e. [DOI] [PubMed] [Google Scholar]
- Eliceiri B., Labella T., Hagino Y., Srivastava A., Schlessinger D., Pilia G., Palmieri G., D'Urso M. Stable integration and expression in mouse cells of yeast artificial chromosomes harboring human genes. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2179–2183. doi: 10.1073/pnas.88.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza D., Ajioka J. W., Burke D. T., Hartl D. L. Mapping the Drosophila genome with yeast artificial chromosomes. Science. 1989 Nov 3;246(4930):641–646. doi: 10.1126/science.2510296. [DOI] [PubMed] [Google Scholar]
- Green E. D., Olson M. V. Chromosomal region of the cystic fibrosis gene in yeast artificial chromosomes: a model for human genome mapping. Science. 1990 Oct 5;250(4977):94–98. doi: 10.1126/science.2218515. [DOI] [PubMed] [Google Scholar]
- Huxley C., Hagino Y., Schlessinger D., Olson M. V. The human HPRT gene on a yeast artificial chromosome is functional when transferred to mouse cells by cell fusion. Genomics. 1991 Apr;9(4):742–750. doi: 10.1016/0888-7543(91)90369-p. [DOI] [PubMed] [Google Scholar]
- Lander E. S., Waterman M. S. Genomic mapping by fingerprinting random clones: a mathematical analysis. Genomics. 1988 Apr;2(3):231–239. doi: 10.1016/0888-7543(88)90007-9. [DOI] [PubMed] [Google Scholar]
- Little R. D., Porta G., Carle G. F., Schlessinger D., D'Urso M. Yeast artificial chromosomes with 200- to 800-kilobase inserts of human DNA containing HLA, V kappa, 5S, and Xq24-Xq28 sequences. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1598–1602. doi: 10.1073/pnas.86.5.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick M. K., Shero J. H., Cheung M. C., Kan Y. W., Hieter P. A., Antonarakis S. E. Construction of human chromosome 21-specific yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9991–9995. doi: 10.1073/pnas.86.24.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanaro V., Casamassimi A., D'Urso M., Yoon J. Y., Freije W., Schlessinger D., Muenke M., Nussbaum R. L., Saccone S., Maugeri S. In situ hybridization to cytogenetic bands of yeast artificial chromosomes covering 50% of human Xq24-Xq28 DNA. Am J Hum Genet. 1991 Feb;48(2):183–194. [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Ledbetter S. A., Corbo L., Victoria M. F., Ramírez-Solis R., Webster T. D., Ledbetter D. H., Caskey C. T. Alu polymerase chain reaction: a method for rapid isolation of human-specific sequences from complex DNA sources. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6686–6690. doi: 10.1073/pnas.86.17.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum R. L., Airhart S. D., Ledbetter D. H. A rodent-human hybrid containing Xq24-qter translocated to a hamster chromosome expresses the Xq27 folate-sensitive fragile site. Am J Med Genet. 1986 Jan-Feb;23(1-2):457–466. doi: 10.1002/ajmg.1320230137. [DOI] [PubMed] [Google Scholar]
- Reilly D. S., Lewis R. A., Nussbaum R. L. Genetic and physical mapping of Xq24-q26 markers flanking the Lowe oculocerebrorenal syndrome. Genomics. 1990 Sep;8(1):62–70. doi: 10.1016/0888-7543(90)90226-k. [DOI] [PubMed] [Google Scholar]
- Schlessinger D. Yeast artificial chromosomes: tools for mapping and analysis of complex genomes. Trends Genet. 1990 Aug;6(8):248, 255-8. doi: 10.1016/0168-9525(90)90207-m. [DOI] [PubMed] [Google Scholar]
- Sealey P. G., Whittaker P. A., Southern E. M. Removal of repeated sequences from hybridisation probes. Nucleic Acids Res. 1985 Mar 25;13(6):1905–1922. doi: 10.1093/nar/13.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman G. A., Jockel J. I., Domer P. H., Mohr R. M., Taillon-Miller P., Korsmeyer S. J. Yeast artificial chromosome cloning of a two-megabase-size contig within chromosomal band 18q21 establishes physical linkage between BCL2 and plasminogen activator inhibitor type-2. Genomics. 1991 Feb;9(2):219–228. doi: 10.1016/0888-7543(91)90245-a. [DOI] [PubMed] [Google Scholar]
- Silverman G. A., Ye R. D., Pollock K. M., Sadler J. E., Korsmeyer S. J. Use of yeast artificial chromosome clones for mapping and walking within human chromosome segment 18q21.3. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7485–7489. doi: 10.1073/pnas.86.19.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M., Little R. D., Abidi F., Porta G., Labella T., Cooper T., Della Valle G., D'Urso M., Schlessinger D. Human Xq24-Xq28: approaches to mapping with yeast artificial chromosomes. Am J Hum Genet. 1990 Jan;46(1):95–106. [PMC free article] [PubMed] [Google Scholar]